|
Ornithology Parental Care |
|
Ornithology Parental Care |
At hatching, some young birds are entirely dependent on their
parents, while others are able to leave the nest and begin finding their
own food within hours of hatching. Based on such differences, young birds
are generally categorized as either altricial or precocial.

The altricial–precocial developmental spectrum in birds. Orange = life in the egg; light green = life in the nest, outside the egg;
dark green = terrestrial/aquatic life outside the nest; blue = aerial life. *Not all species exhibit a post-fledging dependence period, e.g., megapodes (From: Ruaux et al. 2020).

Malleefowl (Leipoa ocellata) chick
(Source: http://abc.net.au/science/scribblygum/October2000/gallery.htm)
Brush Turkey - a megapode
Young Wood Ducks leaving the nest
Great Crested Grebes and young
Black Skimmer nesting (with semiprecocial young)


Young American Robins
Summary of characteristics of young birds at hatching (Nice 1962):
|
Development |
present? |
open? |
|
Themselves? |
present? |
|
| Superprecocial |
|
|
|
|
|
|
| Precocial |
|
|
|
|
|
|
| Subprecocial |
|
|
|
|
|
|
| Semiprecocial |
|
|
|
|
|
|
| Semialtricial 1 |
|
|
|
|
|
|
| Semialtricial 2 |
|
|
|
|
|
|
| Altricial |
|
|
|
|
|
|
| A 121 million-year-old baby arboreal bird, fossilized in its egg, found in China (Zhou and Zhang 2004) -- The fossil has piqued researcher's interest because it had feathers, whereas many modern birds are naked and helpless when they hatch. The researchers know the bird, found in northeast China, was an embryo because the fossil is tucked up in very characteristic way for an unhatched chick. "The tucked posture of the fossil is consistent with a late-stage embryo rather than with a hatchling, in which case the head would have raised beyond the vicinity of the feet," said authors Zhonghe Zhou and Fucheng Zhang. The interesting thing about this bird is that for something that has not yet hatched, it is almost fully formed, meaning the bird must have been prococial. Most modern arboreal (tree dwelling) birds are altricial so they can grow to almost full-size in a protected environment, like a nest, before they must attempt the risky business of flying. The fact that this bird - which lived during the Lower Cretaceous - was precocial suggests it did not have the same luxury as its modern day counterparts. It may have been forced to make its own way in the world much sooner. "The fact that its feathers were so well developed could mean that these things could fly quite soon after they hatched," said Dr Milner of London's Natural History Museum. This fossil supports the notion that the first birds had not yet developed the altricial strategy, with all the intensive parental care that it entails. "It is generally believed that precociality is ancient and altriciality is derived," said Dr Zhou and Dr Zhang in their paper. |

The bird was preserved in a space about 1.4 by 0.8 inches (35 by 20 millimeters)
|
Precocial development is the primitive, or original, mode, with the
altricial mode developing independently in several groups of birds (Ricklefs
1983, Gill 1995). "Precociality puts a premium on the ability of females
to obtain abundant resources before laying. They must produce energy-rich eggs to support the greater in-egg development
of the chicks (eggs of precocial birds may contain almost twice the calories
per unit weight as those of altricial birds). Females of altricial species
do not have such large nutritional demands before egg laying, but must
be able (with their mates) to find sufficient food to rush their helpless
young (see drawing to the right) through to fledging. While the young are
in the nest, the entire brood is extremely vulnerable to predation and
is dependent on concealment of the nest and parental defense for survival.
In contrast, precocial young, having left the nest, have some ability to
avoid predation, and there is a much smaller chance of the entire brood
(as opposed to single chicks) being devoured. Interestingly, there seems
to be an evolutionary trade-off in bird brain sizes related to the degree
of precocity. Precocial species have relatively large brains at hatching-as
one might expect since the young, to one degree or another, must be able
to fend for themselves. But precocial species trade for this advantage
an adult brain that is small in relation to their body size. Altricial
young, in contrast, are born small-brained, but on the protein-rich diet
provided by the adults (and with their highly efficient digestive tracts)
postnatal brain growth is great, and the adults have proportionally larger
brains than precocial species" (Ehrlich et al. 1988).
must produce energy-rich eggs to support the greater in-egg development
of the chicks (eggs of precocial birds may contain almost twice the calories
per unit weight as those of altricial birds). Females of altricial species
do not have such large nutritional demands before egg laying, but must
be able (with their mates) to find sufficient food to rush their helpless
young (see drawing to the right) through to fledging. While the young are
in the nest, the entire brood is extremely vulnerable to predation and
is dependent on concealment of the nest and parental defense for survival.
In contrast, precocial young, having left the nest, have some ability to
avoid predation, and there is a much smaller chance of the entire brood
(as opposed to single chicks) being devoured. Interestingly, there seems
to be an evolutionary trade-off in bird brain sizes related to the degree
of precocity. Precocial species have relatively large brains at hatching-as
one might expect since the young, to one degree or another, must be able
to fend for themselves. But precocial species trade for this advantage
an adult brain that is small in relation to their body size. Altricial
young, in contrast, are born small-brained, but on the protein-rich diet
provided by the adults (and with their highly efficient digestive tracts)
postnatal brain growth is great, and the adults have proportionally larger
brains than precocial species" (Ehrlich et al. 1988).
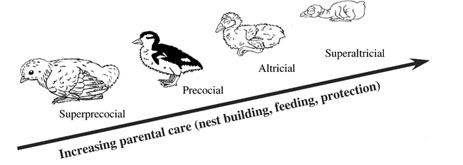
Spectrum of developmental stages from super-precocial brush turkeys to super-altricial songbirds. Parental care necessarily varies with the different categories of development at hatching. Precocial and superprecocial birds are characterized by patterns of simple parental care, minimal nest attendance, and simple nest structure; all those features are considered phylogenetically primitive. Galliformes and Anseriformes seek their own food the day that they hatch but depend on parents for some degree of brooding and protection. On the other hand, altricial species are characterized by sophisticated parental care that includes complex nest building and high attendance to the offspring. Those traits are associated with altricial development (e.g. complex nest construction and strong parental care) and are also correlated with an increase in range of flight styles, flight speeds, and ecological habits (Dial 2003). |
After hatching, avian parental care may involve brooding and feeding nestlings as well as protecting young from predators. Only a few species (brood parasites and the moundbuilders or megapodes) exhibit no post-hatching parental care. For those that provide care:
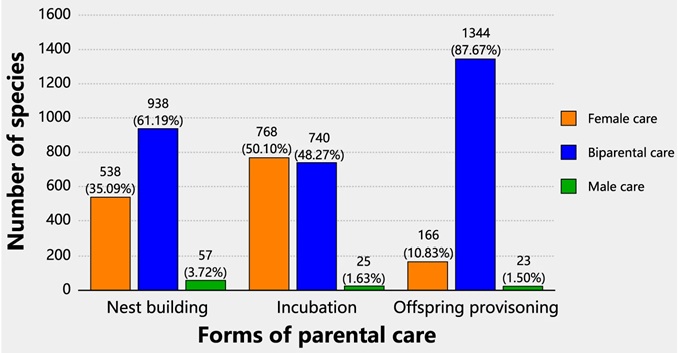
Forms of parental care based on 1533 species with full data on care pattern (Female care, Biparental care,
and Male care) across three forms of parental care: nest building, incubation, and offspring provisioning. Bars of
different colors represent different categories of care patterns. Note that a few species of cooperative breeding
were grouped into the Biparental care category (N = 56 species in nest building, N = 48 in incubation, and
N = 139 in offspring provisioning) (From: Wang et al. 2023).
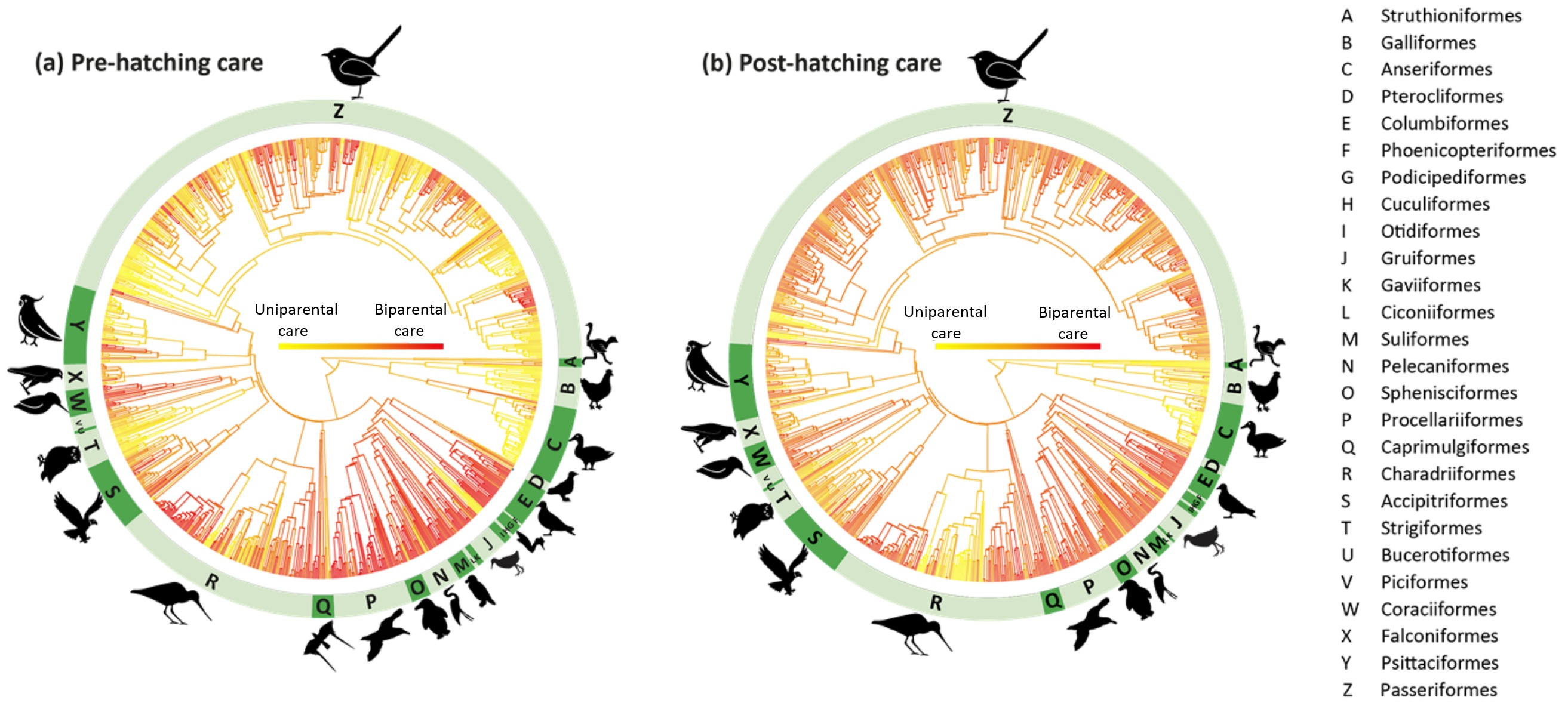
Phylogenetic distribution of parental cooperation in (a) prehatching care and (b) post-hatching care based on 1065 and 991 bird species, respectively.
Red=egalitarian biparental care, yellow=uniparental care. Long et al.'s (2022) analysis revealed that parental care strategies are influenced by coloniality
(solitary, semi-colonial, colonial) and chick development mode (altricial vs. precocial). Colonial and altricial species provide more
biparental care than
solitary and precocial species, respectively. In contrast, food type (plant, invertebrate, vertebrate), nest structure (open vs. closed), and body size do not
covary systematically with parental care patterns in birds. Taken together,
these results suggest that living in groups and/or having high-demand offspring
are strongly associated with biparental care.
Altricial young are unable to control their body temperature
and
must be kept warm when ambient temperatures are low or cool when a nest
is in direct sunlight. Parents help nestlings maintain their body temperatures
by brooding - covering them to keep them warm or shielding them from the
sun or rain. The duration of the brooding period depends on (Pettingill
1985):
Timing of endothermy in (Top) a precocial species (Mallard) and (Bottom) an altricial species (From: Price and Dzialowski 2018).
Brooding effort relative to nestling age for three species of altricial birds. (a, b) an open-nesting species (Ochraceous Bulbul,
Alophoixus ochraceus), (c, d) an enclosed-nesting species (Bornean Stubtail, Urosphena whiteheadi), and (e, f) a cavity-nesting
species (Mountain Chickadee, Poecile gambeli). Lines are model-estimated rates of decline in brooding effort with age of young,
with 95% confidence intervals, and lines ending at fledging age. Initial magnitude is the modelled % of time spent brooding on
hatch day (day 0) and b is the modelled rate of decrease in brooding effort with age of the young.
 |
European Shag (Phalacrocorax aristotelis) nestlings
exhibited an incipient endothermic response when 9 days old, and became
homeothermic at ages of 15–18 days. The index of homeothermy (HI) was calculated
by dividing the final temperature differences between the nestlings and
their surroundings by the initial temperature differences, using the formula:
HI = (Tf - Ta)/(Ti - Ta) where Tand Ti are final and initial body temperatures, respectively, and Ta is ambient temperature. The index is equal to 1 when Tb is maintained without change, and 0 when Tb falls to Ta within 45 min (Østnes et al. 2001). |
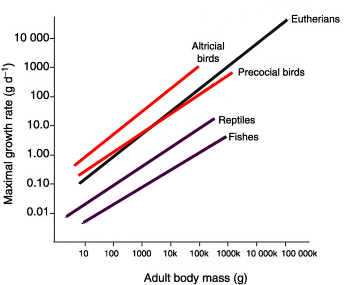
| Growth rates of passerines -- The reasons why growth and developmental rates vary widely among species have remained unclear. Previous examinations of possible environmental influences on growth rates of birds yielded few correlations, leading to suggestions that young may be growing at maximum rates allowed within physiological constraints. However, estimations of growth rates can be confounded by variation in relative developmental stage at fledging. Remes and Martin (2002) re-estimated growth rates to control for developmental stage. They used these data to examine the potential covariation of growth and development with environmental variation across a sample of 115 North American passerines. Contrary to previous results, Remes and Martin (2002) found that growth rates of altricial nestlings were strongly positively correlated to daily nest predation rates, even after controlling for adult body mass and phylogeny. In addition, nestlings of species under stronger predation pressure remained in the nest for a shorter period, and they left the nest at lower body mass relative to adult body mass. Thus, nestlings both grew faster and left the nest at an earlier developmental stage in species with higher risk of predation. Growth patterns were also related to food (aerial foragers tend to have slower growth rates), clutch size (growth rates are slower in species with larger clutches), and latitude (faster growth at higher latitudes). These results support a view that growth and developmental rates of altricial nestlings are strongly influenced by the environmental conditions experienced by species (Figure from Erickson 2005). |
Feeding Young
Among altricial (and semi- and subprecocial) species of birds, one or both adults begin to feed young (or show young where food can be obtained) soon after hatching. In most socially monogamous species plus some polygynous species, both sexes help provision the young. Among birds that deliver food to young, food may be transferred from adult to young in several different ways (Pettingill 1985):
Osprey feeding young
House Wren nest
Feeding visits to nests are typically quick (less time at the nest means less activity that might attract the attention of predators). However, particularly early in the nestling period, females may remain at the nest after feeding nestlings to brood the young. After feeding nestlings, adults often pick up fecal sacs (packages of excrement surrounded by a gelatinous membrane; see photo of a Great Tit fecal sac below) that may be eaten (particularly when nestlings are very young) or carried from the nest for disposal. Older nestlings may 'shoot' their feces away from the nest (e.g., see Bald Eagle video below).

Used with permission of Takashi Koike
Source: http://www.fsinet.or.jp/~bird/bird/greattit/kara96.html
American Robins and fecal sacs
Adult American Redstart feeds young and removes fecal sac from nest
Nestling Bald Eagle defecates
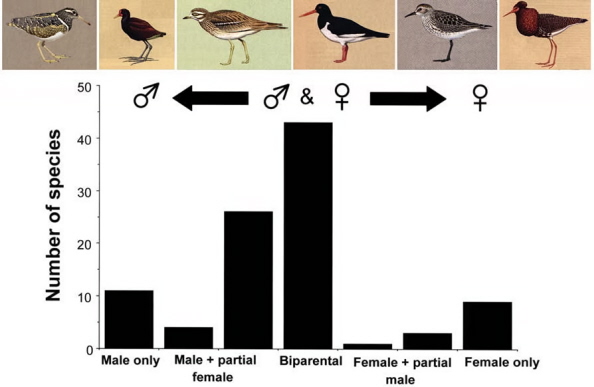
Distribution of parental care in shorebirds. Male-only care is often associated with polyandrous mating systems, whereas female-only care is
associated with polygyny and leks (modified from Székely and Reynolds 1995). The species pictured above the graph are, from left to right, the
Greater Painted Snipe (Rostratula benghalensis), Wattled Jacana (Jacana jacana), Eurasian Thick-knee (Burhinus oedicnemus), Eurasian
Oystercatcher (Haematopus ostralegus), White-rumped Sandpiper (Calidris fuscicollis), and Ruff (Philomachus pugnax).
Source: Szekely et al. (2006).
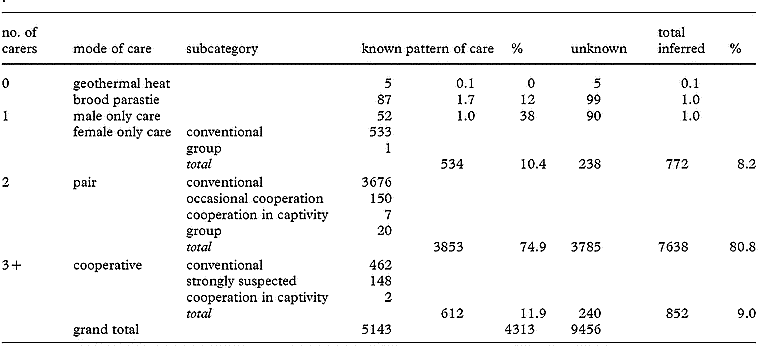
Modes of parental care (Cockburn 2006) . -- Estimates of the incidence of major classes of parental care by birds have been drawn from classical studies that preceded both the publication of a massive secondary literature and the revolution driven by molecular approaches to avian phylogeny. Cockburn (2006) reviewed this literature in the light of new phylogenetic hypotheses and estimated the prevalence of six distinct modes of care: use of geothermal heat to incubate eggs, brood parasitism, male only care, female only care, biparental care, and cooperative breeding. Female only care and cooperative breeding are more common than has previously been recognized, occurring in 8 and 9% of species, respectively (see Table below). Biparental care by a pair bonded male and female is the most common pattern of care, but, at 81% of species, the pattern is less common than once believed (see Table below).
The number of bird species known and inferred to exhibit different modes of parental care.
Male only care. It has proved difficult to identify a common pattern between the groups where males are the predominant carers. Even the best-known correlate, with precocial young, is now known to have at least one exception (Andersson 1995). Owens (2002) has argued that contrasts between families exhibiting male and female only care support a low-density hypothesis, which proposes that males should care if density is sufficiently low to prevent them gaining any benefit by desertion, as they are unlikely to find alternative mates. The basis for this contrast is motivated by the dynamic desertion strategy of Holarctic waders. However, it is unclear why the low-density hypothesis favors female desertion relative to biparental care. Clearly, a single hypothesis is unlikely to encompass all cases of male only care.
Female only care. There is abundant evidence that common selection pressures have driven convergent evolution of female-only care. There are numerous origins of female-only care among taxa with nidicolous, altricial young. In such taxa, female-only care has evolved in birds that feed largely on tropical fruit and nectar. The correlation has been explained in complementary ways from female and male perspectives. Because tropical fruit and flowers can be massively abundant, yet availability can be patchy on short-term spatial and temporal scales, males may gain advantage from the defense of fruiting trees or geographical locations that females frequently traverse in order to find fruiting or flowering trees (the hotspot hypothesis). From the female perspective, the limitation on reproduction is likely to be associated with the ability of the young to extract nutrition from abundant but low quality food. Hence male care is of limited value, allowing females to choose freely among males for good genes rather than for direct benefits from the male such as a high quality territory or paternal provisioning (the constrained female hypothesis).
What about primarily insectivorous taxa, where male care should be at a premium? Many insectivorous taxa with female-only care occur in dense nesting aggregations in rich marshlands in which high abundance of food occurs because of seasonal irruptions of aquatic insects. This reduces the need for females to obtain care and together with high female densities, facilitates the evolution of polygyny. However, a variety of taxa cannot be explained via this approach, particularly some insectivorous denizens of rainforests. Predation might be important in these species because, as originally suggested for frugivores, males might enhance detection of the nest by predators because any attempt by males to guard a single female against extra-pair mating would impose impossible costs from the sit-and-wait predators that predominate in rainforest interiors and because the intrinsic mortality schedules of long-lived tropical species may make parents reluctant to take risk during reproduction. Further investigations of these cases will be extremely valuable.
Cooperative breeding. In the 1097 species of New World suboscines, cooperative breeding is rare, inferred in just 16 species. By contrast, a larger proportion of oscines are cooperative breeders (577 of 4456 species; 13%). It is unlikely that there is a simple ecological or life history explanation for this difference. Both clades have diversified into an enormous range of niches and show overlapping variation in life history. The low prevalence in suboscines is unlikely to be a result of the environment they occupy. Several oscine taxa have primarily radiated in the Neotropics and hence overlap the range of the New World suboscines. Many of these have a high incidence of cooperation (e.g., New World jays, mimids, emberizids, icterids and wrens).
| Red or Dead -- As any springtime nest attests, many young birds beg for food by making lots of noise & opening their beaks wide to reveal brightly colored 'gapes'. Among Barn Swallows (Hirundo rustica) this familiar scene hides an example of the harshness of life (Saino et al. 2000). Apparently, parent swallows apportion food according to the how healthy they judge nestlings to be. And only the fit get fed. On what do adult birds base this health audit? The redness of the wide-open beaks they are confronted with. If food is short, no red gape can mean no dinner for a needy nestling. Saino and colleagues drew this conclusion from experiments with baby Barn Swallows. First they tricked adult birds by dyeing some of their nestlings' gapes with red food coloring. These impostors got more food. Then Saino's group found that when they challenged the immune systems of swallow nestlings (with sheep red blood cells), the color of the birds' gapes dulled -- sometimes even becoming green or yellow -- and they were overlooked by their parents. But why? Pigments called carotenoids are largely responsible for gape hue, especially one called 'lutein'. In birds and mammals, carotenoids also play a role in stimulating and regulating the immune system. So Saino's group wondered whether swallows whose immune systems are wrestling with something -- a bacterial infection, say -- have dowdier gapes because they cannot spare carotenoids for the less important business of imbuing the inner lining of their throats with an alluring blush. They tested this by giving extra lutein to swallows infected with sheep red blood cells. These lutein-boosted, but nonetheless ailing nestlings, developed gapes just as red - and were fed equally well by their parents - as their healthy siblings. This indicates that parent Barn Swallows, anxious to make the best use of limited food resources, use gape color as, "a reliable signal of offspring quality." It's a jungle out there. -- Sara Abdulla, Nature Science Update |
 Source:
Source:
cspottiswoode.free.fr/Anders/Research.htm |
Young Barn Swallows being fed
Parental conflict in birds -- Parents often conflict over how much care to provide to their offspring because care requires time and energy and reduces parental survival and opportunities for additional mating opportunities or polygamy. Therefore, each parent prefers the other to provide more care. This conflict is expected to produce a negative relationship between male and female parental care, the strength of which may be mediated by both ecological and life-history variables. In a broad-scale comparative study of parental conflict using 193 species from 41 families of birds, Olson et al. (2008) found that male and female parental care were negatively correlated across a broad range of bird taxa. This indicates that there is an evolutionary tug-of-war between males and females over the care of offspring, a result consistent with the prediction of sexual conflict theory. Olson et al. (2008) found strong influences of both male and female mating opportunities on patterns of parental care disparity. In addition, analysis revealed that the developmental mode of the young has a strong influence on the relationship between parental care and mating opportunities. In altricial species, males appear not to take into account their level of care relative to that of the female when making decisions regarding additional mating opportunities, whereas in precocial species, males trade off care and polygyny. Interestingly, females were also responsive to mating opportunities: in altricial species, females take on relatively more of the parental care with increased female mating opportunities, while in precocial species, females take on relatively less. Taken together, the sexes appear to play the same strategies, at least in birds: if their young need little care, then both males and females respond to enhanced mating opportunities. Taken together, these results suggest that sexual conflict is a key element in the evolution of parental care systems. They also support the view that the major correlates of the intersexual conflict are mating opportunities for both sexes.
 Sonagrams (2.5 sec) of the begging calls of (a) a single Reed Warbler, (b) a brood of four Reed Warblers, (c) a single cuckoo nestling, and (d) a single European Blackbird nestling. |
|
Manipulative begging by parasitic cuckoo chicks (Lotem 1998) -- The Common Cuckoo is an obligate brood parasite that lays a single
egg in the nests of several passerine host species. Soon after hatching, the cuckoo nestling ejects the host eggs or young from the nest and is raised
alone. Previous studies have shown that a single cuckoo chick raised by a small passerine, such as the Reed Warbler, is fed at the same rate as, and
for a longer period than, a brood of four host young. One suggestion to explain the success of the cuckoo chick in eliciting parental care was that its
large size, bright gape and intense begging provided the parents with a super normal stimulus or with an image of an especially high quality offspring.
However, Davies et al. (1998) showed that large size alone was insufficient to stimulate adequate provisioning. When they replaced a Reed Warbler
brood with a single European Blackbird chick (Turdus merula) or a Song Thrush (T. philomelos), which are similar in size to the cuckoo chick, the rate
of food
delivery was significantly smaller than to a single cuckoo chick and similar to a single Reed Warbler chick. Further exploration suggested that the
stimulus
used by cuckoo chicks to elicit host care is their unusual begging call, which, to human ears, sounds remarkably like the begging calls of a whole
brood
of Reed Warblers. Indeed, on a sonogram (above), the cuckoo begging calls and those of a whole brood of Reed Warblers are very similar. In
contrast,
blackbird and
thrush chicks have calling rates of only about one call per second, which could explain their inability to elicit the same provisioning rate
as a single
cuckoo,
despite being the same size. Thus, a cuckoo chick needs vocal trickery to compensate for the fact that it presents a visual stimulus of just
one gape and
the
cuckoo's way of deceiving its host is to pretend to be a group of several offspring rather than appearing as a single high quality one.
When a parent arrives at the nest (e.g., an adult Velvet Asity, Philepitta castenea, visiting its nest; Williams 2001), young typically respond (although to varying degrees depending on how hungry they are) by begging --- uttering 'begging' calls with mouth wide open. In many species, young have brightly colored mouth linings to help parents direct the food into their mouths.
Feeding rates typically increase with the increasing age of nestlings. Parents may make more trips to the nest, deliver more food per trip or both. Of course, the frequency of provisioning visits also varies with species and number of young. For example (Welty and Baptista 1988):
Nestling Northern Cardinals begging
Begging attracts predators -- Begging by nestling birds has been used to test evolutionary models of signalling but theory has outstripped evidence. Eavesdropping predators potentially impose a cost on begging that ensures signal honesty, yet little experimental evidence exists for such a cost at active nests because the use of artificial nests, long playback bouts and absence of parents may have exaggerated costs. Haff and Magrath (2011) broadcast short periods (1 hr) of either nestling vocalizations or background noise at active White-browed Scrubwren (Sericornis frontalis) nests. Nestlings called naturally during both treatments, allowing the investigators to test whether elevated calling increases risk, a key but rarely tested assumption of evolutionary models. Predators visited nests exclusively during periods of elevated calling. Furthermore, playbacks affected neither adult visits nor nestling activity, suggesting that calling alone attracted predators. Adults gave alarm calls and nestlings usually called less when predators approached nests. Predation risk to broods is, therefore, likely to fluctuate substantially over short periods of time, depending on nestling hunger and whether adults or young have detected predators. These results confirms a present-day cost of nestling begging, demonstrates that this cost can be incurred over short periods and supports the importance of parent–offspring antipredator strategies in reducing predation risk.
Natal plumage of a just-hatched Cinereous Mourner (Laniocera hypopyrra; family Tityridae) consists of bright orange down feathers with bright white tips, This plumage, in combination with side-to-side head movements when disturbed, means that these young nestlings resemble a hairy, aposematic caterpillar. This appears to be an example of Batesian mimicry, resembling a noxious prey item to avoid predation, and may enhance survival for a tropical songbird with a relatively long nestling period for its size (20 days)
| Owl chicks are blessed with impeccable manners - Most scientists have assumed that nestlings call to attract the attention of their parents. But in some species, chicks call even when parents aren't around. Alexandre Roulin wondered whether these nestlings were communicating with each other. So, Roulin et al. (2000) chose two siblings at random from broods of Barn Owls (Tyto alba) and gave one of the chicks dead mice to eat during the day. They found that the hungry nestling cried more often during the following night than the chick that had eaten. But once the hungry chick had been fed, its sibling started to beg more. In another experiment, the more siblings in a nest, the less the chicks called. It's not what you'd expect if the chicks were trying to shout each other down. The team think the chicks don't beg when they have little chance of getting food. "If one nestling is more hungry, the value of the food for it is higher", explains Roulin. "A hungry nestling will fight physically for the prey." So, it's not worthwhile for the less hungry nestling to compete for food it is unlikely to win. Instead, chicks monitor each other's hunger levels by the intensity of each other's cries; less hungry birds back down, saving energy and waiting their turn. Because nobody has previously considered that nestlings might communicate in the absence of parents, it's possible that nestlings of other species behave in the same way. |

See a Barn Owl video courtesy of the BBC |
Most
parent birds feed their young the same food that they eat: insect eaters
feed their young  insects, fish eaters bring fish, seed eaters bring seeds, and so on. However,
seed-eaters and fruit-eaters may also provide their young with some insects
(which contain more protein) (Skutch 1976). Parents also vary the size
of food items, typically bringing smaller items to very young (and small)
nestlings and larger items to larger, older young. The food given to young
birds contains all the moisture they need, and parents do not bring them
water to drink (Skutch 1976). Notable exceptions are the sandgrouse
(e.g., the Black-bellied Sandgrouse to the right) --- parents (particularly
males) have specially modified abdominal feathers with great water holding
capacity. In fact, these feathers have structural modifications (the barbules
are not hooked together) that make them three to four times more absorbent
than synthetic sponges (del Hoyo 1987). Adults may travel great distances
to water, soak up the water in the abdominal feathers, then fly back to
the nest so their young can drink the water from their plumage (Skutch
1976).
insects, fish eaters bring fish, seed eaters bring seeds, and so on. However,
seed-eaters and fruit-eaters may also provide their young with some insects
(which contain more protein) (Skutch 1976). Parents also vary the size
of food items, typically bringing smaller items to very young (and small)
nestlings and larger items to larger, older young. The food given to young
birds contains all the moisture they need, and parents do not bring them
water to drink (Skutch 1976). Notable exceptions are the sandgrouse
(e.g., the Black-bellied Sandgrouse to the right) --- parents (particularly
males) have specially modified abdominal feathers with great water holding
capacity. In fact, these feathers have structural modifications (the barbules
are not hooked together) that make them three to four times more absorbent
than synthetic sponges (del Hoyo 1987). Adults may travel great distances
to water, soak up the water in the abdominal feathers, then fly back to
the nest so their young can drink the water from their plumage (Skutch
1976).
Female Anna's Hummingbird feeding nestlings
Sandgrouse collecting water in its feathers
Adult provisioning and fledgling plumage -- Ultraviolet (UV) reflectance has been implicated in mate selection. Yet, in some bird species the plumage of young varies in UV reflectance already in the nest and long before mate choice and sexual selection come into play. Most birds molt the juvenile body plumage before reaching sexual maturity, and thus, some conspicuous traits of the juvenile body plumage may rather have evolved by natural selection, possibly via predation or parental preference. This second hypothesis is largely untested and predicts a differential allocation of food between fledging and total independence, which is a time period of 2–3 weeks where offspring mortality is also highest. Tanner and Richner (2008) tested the prediction that parents use the individual variation in UV reflectance among fledglings for differential food allocation. They manipulated UV reflectance of the plumage of fledgling Great Tits (Parus major) by treating chest and cheek feathers with a lotion that either did or did not contain UV blockers and then recorded food allocation by parents in an outdoor design simulating postfledging conditions. The visible spectrum was minimally affected by this treatment. Females were found to feed UV-reflecting offspring preferentially, whereas males had no preference. This is the first evidence showing that the UV reflectance of the feathers of young birds has a signaling function in parent–offspring communication and suggests that the UV traits evolved via parental preference.
Carolina Wren nestlings being fed by adults
More spiders = smarter young -- Early nutrition shapes life history. Parents should, therefore, provide a diet that will optimize the nutrient intake of their offspring. In a number of passerines, there is an often observed, but unexplained, peak in spider provisioning during chick development. Arnold et al. (2007) showed that the proportion of spiders in the diet of nestling Blue Tits (Cyanistes caeruleus) varies significantly with the age of chicks but is unrelated to the timing of breeding or spider availability. Moreover, this parental prey selection supplies nestlings with high levels of taurine particularly at younger ages. This amino acid is known to be both vital and limiting for mammalian development and consequently found in high concentrations in placenta and milk. Based on the known roles of taurine in mammalian brain development and function, Arnold et al. (2007) then asked whether by supplying taurine-rich spiders, avian parents influence the stress responsiveness and cognitive function of their offspring. To test this, wild Blue Tit nestlings were provided with either a taurine supplement or control treatment once daily from the ages of 2-14 days. Then pairs of size- and sex-matched siblings were brought into captivity for behavioral testing. Juveniles that had received additional taurine as neonates took significantly greater risks when investigating novel objects than controls. Taurine birds were also more successful at a spatial learning task than controls. Additionally, those individuals that succeeded at a spatial learning task had shown intermediate levels of risk taking. Non-learners were generally very risk-averse controls. Early diet therefore has downstream impacts on behavioural characteristics that could affect fitness via foraging and competitive performance. Fine-scale prey selection is a mechanism by which parents can manipulate the behavioral phenotype of offspring.
Life-history aspects of family strife (Forbes
and Mock 2000) -- Breeding birds have evolved life-history traits that
tend to maximize lifetime reproductive success. Within this broad pattern,
many variations are possible because all traits are co-evolved with numerous
others in complex ways. Clutch-size, for example, is frequently lower than
the number of young parents are capable of supporting by working at their
top capacity, especially in long-lived species. Nevertheless, studies of
species with fatal competition among nestmates (i.e., siblicide) have shown
that parents routinely create one offspring more than they normally will
raise, as if counting on brood-reduction to trim family size after hatching.
Three general and mutually compatible parental incentives for initial over-production
have been identified:
|
In obligate brood-reducing species,
hatching asynchrony is typically highly exaggerated, such that first-hatched
nestlings enjoy an almost insuperable competitive edge. In siblicidal birds
(where brood reduction is substantially caused by sibling aggression),
including various pelicans, eagles, boobies, egrets, and cranes, the marginal
nestling is typically bludgeoned to death at an early age whenever the
full brood hatches. Where nest-mate asymmetries are less extreme, the executions
tend to be more protracted and less certain. In many facultatively siblicidal
birds such as Blue-footed Boobies (Sula nebouxii) and Cattle Egrets
(Bubulcus ibis), the extra nestling is often maintained for days
or even weeks, during which it simultaneously embodies insurance value
(enjoying enhanced survival if eventually predeceased by a senior nestmate)
and the Lack potential of adding to the larger absolute number of independent
offspring if ecological conditions prove generous. The parentally determined
initial competitive asymmetries thus modulate the costs and likelihood
of brood reduction and the duration of insurance coverage. --- Forbes
and Mock (2000).
|
Avian infanticide (From: Kermott et al. 1991) -- Infanticide is recognized as an adaptive behavior which may increase the reproductive success of the perpetrator. Among birds, infanticide is often committed by individuals who replace other individuals on territories containing offspring of the previous resident. Replacement can occur after one member of a pair dies or deserts the territory, or through physical eviction of one member of a pair by a non-resident bird. Two hypotheses for the adaptive value of infanticide in this context have been proposed. First, by eliminating dependent offspring, the perpetrator may shorten the interval between replacement and the time that the resident is capable of starting a new breeding attempt. Second, destruction of offspring may provide the perpetrator access to a resource critical for attracting a mate, such as a nest site. Despite these potential benefits, replacements do not always commit infanticide. In some species, replacements have been observed to act indifferently towards resident offspring or provide them with some form of parental care. Rohwer (1986) outlined conditions under which infanticide, adoption or indifference would maximize the future reproductive success of a replacement male (i.e., by rapid acquisition of a mate). For short-lived species where the potential benefits of a response are not likely to be realized in subsequent breeding seasons, infanticide should be favored when only enough time remains in the breeding season to complete either the resident female's current breeding attempt or one new attempt. Infanticide will also be favored, regardless of the number of broods that can be raised that breeding season, when there is a high probability that the resident female will pair with the replacement male following infanticide, or if there is a high probability that the replacement male can attract a new mate to the territory if the resident female deserts. Indifference or adoption will only be favored over infanticide if the resident female represents the only mate a replacement male is likely to obtain that year, i.e., there must be a high probability that the resident female will desert the replacement male if he kills her offspring, and a low probability that the male will attract a new mate if the resident female deserts. In addition, enough time must remain in the breeding season to complete both the resident female's breeding attempt and at least one additional attempt.

Male (left) and female (right) Eclectus Parrots (Image source: Wikipedia). Females used their bright red color to advertise their presence in nest cavities; the green males blend in with the rainforest canopy while foraging. This difference in the color of males and females also allows females to determine the sex of offspring.
Sex ratio adjustment via sex-specific infanticide -- Infanticide is easiest to understand when it involves killing the offspring of others (as described above), but a parent may also kill its own offspring if the sacrifice of currently dependent young leads to higher survival of brood mates or an improvement in the parent's likely future reproduction. However, sex-specific infanticide by parents of their own offspring, although occurring in some human societies, is rare across species. Its rarity may be because killing one sex combines wasted parental effort with consequent biases in population sex ratios that are detrimental for the fitness of the overproduced sex. Heinsohn et al. (2011) found that killing male offspring can be advantageous to Eclectus Parrot (Eclectus roratus) mothers even though frequency-dependent selection then elevates the reproductive value of sons above that of daughters. In poorer-quality nest hollows, broods with a single female nestling had higher reproductive value than broods in which the female had a younger brother. This is because poor-quality nests often flood, drowning the chicks. Because females typically fledge 7 days earlier than males, females in poor-quality hollows have a better chance of reproductive success if they concentrate their efforts on faster-fledging females. These results demonstrate frequent targeted removal of male nestlings within 3 days of hatching in these specific brood types and nesting conditions. The ability of Eclectus Parrots to perceive the sex of their offspring relatively early may favor decisions to kill one sex before further investment in parental care.
Defending eggs and young
Predators of various types will prey on nestlings, and adults in most species will exhibit some form of defense when potential predators approach a nest. Defense may involve calling, approaching the predator, or, in some cases, even striking the potential predator. Levels of defense by adult birds are typically greater during the nestling period than during incubation. This may be because the reproductive value of young to parents increases as they age. The effectiveness of parental defense varies among species (e.g., birds with 'weapons' like raptors represent a greater threat than birds without such 'weapons') and with the type of predator.
Avian nest defense (snake appears at 1:58)
The broken-wing display is a well-known and conspicuous deceptive signal used to protect birds' broods against diurnal terrestrial predators. Although commonly associated with shorebirds, the display is actually widespread. This behavior has been described in 52 families, suggesting that it independently evolved multiple times. Birds use this display, pretending to be injured, e.g., with a broken wing, to lure predators away from vulnerable eggs or young. Analysis has revealed that birds breeding at higher latitudes or with more exposed nests are more likely to perform the display than tropical birds or species with well-concealed nests (Source: de Framond 2022).
Small Pratincole Broken Wing Display
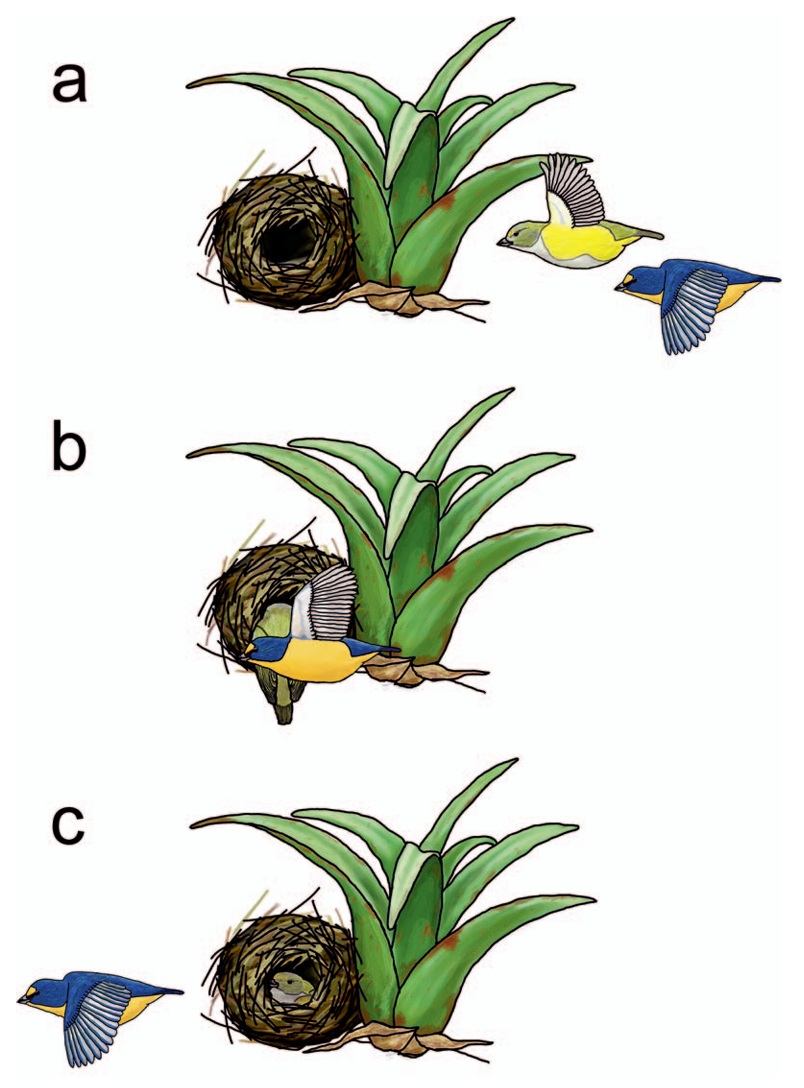
A typical sequence of a mated pair performing coordinated misdirection behavior.
(a) Male and female Yellow-throated Euphonias fly together toward their nest. (b) As one (here, the
female) flies into the nest, the other (here, the male) obscures her from view and flies onward, often changing
direction in flight. (c) One bird enters the nest to incubate, provision nestlings, or brood while the
other bird continues in flight. Human observers tend to visually follow the male in this scenario,
decreasing the probability that they observe the female entering the nest; if visual nest predators are
similarly deceived, coordinated misdirection may decrease nest predation.
Nest predation has driven the evolution of specialized behaviors that decrease the probability that a predator encounters a nest. Gulson-Castillo et al. (2018) collected descriptions from the literature of a behavior where male and female adults fly to their nest as a pair, with one bird flying onward or veering off while the other enters the nest. The most likely function of this behavior is to decrease the risk of nest predation from visual nest predators. In this hypothesis, visual nest predators are distracted by the flying bird and thus fail to observe the bird arriving to the nest entrance (and the nest itself), although the putative adaptive value of this behavior remains to be confirmed. Although this behavior has been sporadically noted in the natural history literature, few ornithologists are aware it is found across multiple taxa, especially in the Neotropics. This behavior has been reported in at least 28 species across five families (and 11 genera) of passerines. Gulson-Castillo et al. refer to this behavior as 'coordinated misdirection' because it depends on the cooperation of at least 2 birds, and its presumed function is a visual misdirection—a ruse to draw an observers’ attention away from the nest.
| Nest defense as parental care -- Intensity of
nest defense against a human intruder was recorded for male and female
Northern Hobbies. Sergio and Bogliani (2001) found that, except during
incubation, intensity of nest defense by females was higher than by males.
For both sexes, defense intensity increased from incubation to fledging,
within the nestling stage, and from fledging to the first 10 days of the
postfledging period. Intensity of nest defense was positively correlated
with brood size in females, but not males. The intensity of defense from
incubation to nestling stages, and even within the nestling period, mirrored
the increase in survival probabilities of the offspring. That is in agreement
with predictions of parental investment theory, based on the growing reproductive
value of the young, due to an increase in expected fitness benefits for
the adults. Female intensity of defense was positively correlated with
brood size. Large broods have greater reproductive value for the parent
than smaller broods, and that may select for higher levels of optimal defense.
|
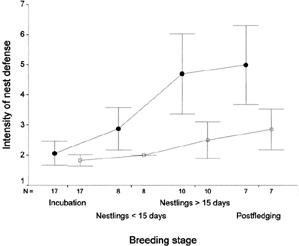
Average intensity of nest defense (±1 SE) by 42 male Northern Hobbies (open square) and 42 female Northern Hobbies (closed circle) during 42 nest defense trials. Intensity of defense against a potential human predator was estimated through an ordinal aggressiveness score ranging from 1 (flies away and disappears) to 8 (stoops closely at the intruder). |
Gray Catbird vs. black rat snake
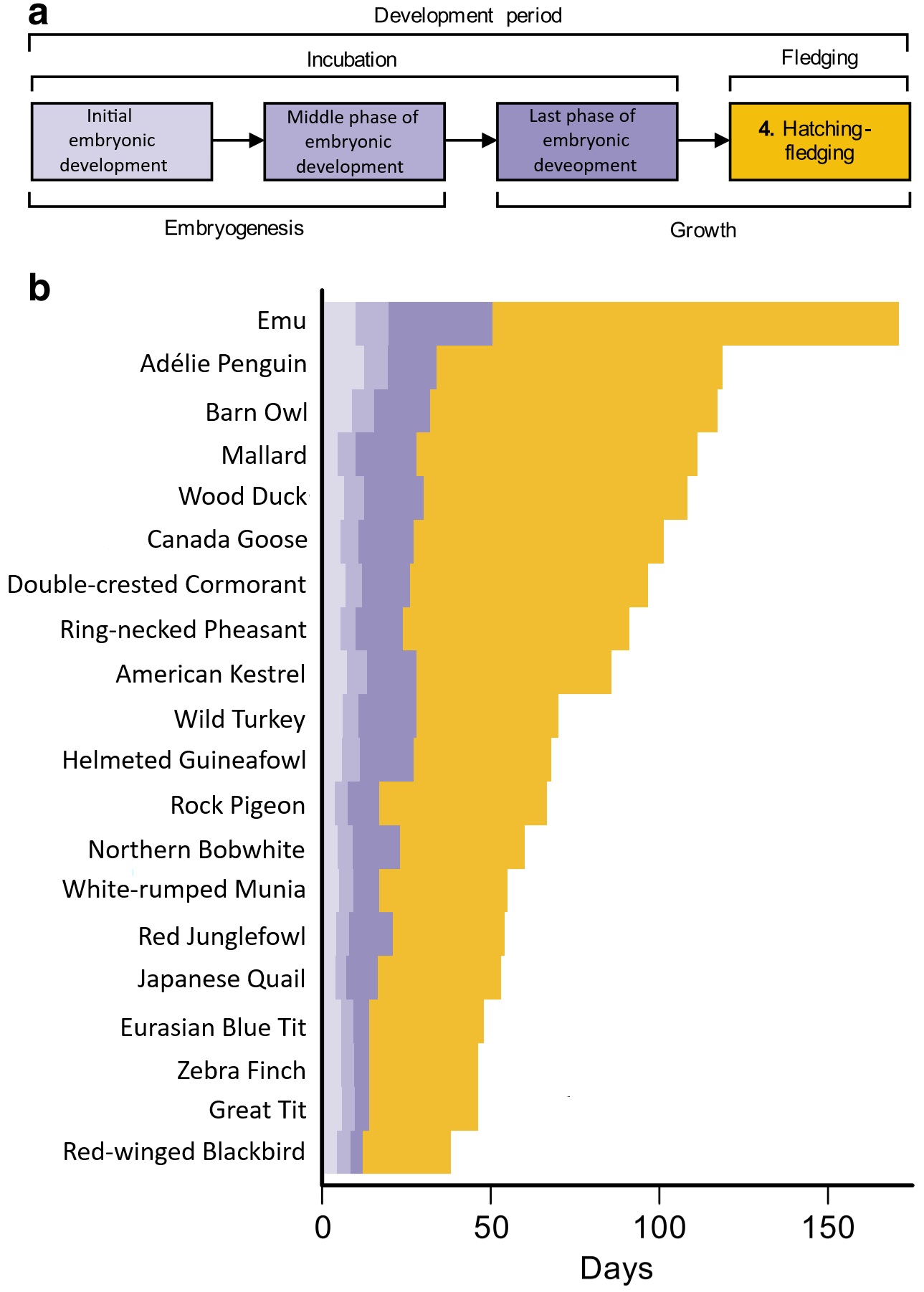
Duration of avian developmental phases. (a) Schematic illustrating dufferent phases of avian ontogeny.
(b) Stacked bar chart showing duration of different developmental stage for different species of birds.
Note that most of the variation is due to differences in the duration of post-hatching parental care. Species
with longer overall developmental durations are generally larger, longer lived, and have smaller clutches (From: Cooney et al. 2020).
Fledging (From: Michaud and Leonard 2000)
Parental care provides obvious benefits to parents through increased growth and survival of young. However, feeding offspring is energetically costly, and protecting them from predators increases the risk of injury or death to the parents. Therefore, parents are expected to care for offspring until the costs of care outweigh the benefits. Because offspring may benefit from care that extends beyond the parental optimum, the length of the dependent period may be a source of conflict for parents and young (Trivers 1974).
In altricial birds, conflict could also occur over the timing of leaving the nest, or fledging. Parents may benefit by decreasing the length of the nestling period and conserving energy for future reproductive attempts and/or migration, while offspring may benefit by extending the nestling period and decreasing their thermoregulatory and activity costs. However, the timing of fledging may not necessarily be a source of conflict. For instance, parents and young may benefit from earlier fledging when the risk of nest predation is high.
Before determining whether conflict over the timing of fledging occurs, it is necessary to understand the factors that influence fledging. At least three factors are potentially relevant:
Finally, competition among nestmates could play a role in the fledging process. In several species, large broods fledge before small broods, apparently because of an increase in competition for food and space in larger broods. Nestmate interactions also could affect individual nestlings differently and thus influence fledging order within broods. For instance, in Marsh Tits (Parus palustris), smaller nestlings initiate fledging under low food conditions, presumably to reduce competition from larger nestmates (Lemel 1989).
Young Dark-eyed Juncos fledge on day 8 post-hatching
Young Allen's Hummingbirds fledging
;
 |
|
Adjustment of pre-fledging mass loss by nestling swifts preparing for flight -- Nestling birds often maintain nutritional reserves to ensure continual growth during interruptions in parental provisioning. However, mass-dependent flight costs require the loss of excess mass before fledging. Wright et al. (2006) tested whether individual variable mass loss prior to fledging is controlled through facultative adjustments by nestlings, or whether it reflects physiologically inflexible developmental schedules. They found that in the face of natural and experimental variation in nestling body mass and wing length, Common Swifts (Apus apus) always achieve very similar wing loadings (body mass per wing area) prior to fledging, presumably because this represents the optimum for flight. Experimental weights (approx. 5% body mass) temporarily attached to nestlings caused additional reductions in mass, such that final wing loadings still matched those of control siblings. Experimental reductions in nestling wing length (approx. 5% trimmed from feather tips) resulted in similar additional mass reductions, allowing wing loadings at fledging to approach control levels. Nestlings may assess their body mass relative to wing area via wing flapping and special 'push-ups' (on the tips of extended wings) performed in the nest. Thus, by facultatively adjusting body mass, but not wing growth, nestling swifts are always able to fledge with aerodynamically appropriate wing loadings.
Common Swift nest, eggs to fledging
Literature cited:
Andersson, M. 1995 Evolution of reversed sex roles, sexual size dimorphism, and mating system in coucals (Centropodidae, Aves). Biol. J. Linn. Soc. Lond. 54: 173-181.
Arnold, K. E., S. L. Ramsay, C. Donaldson, and A. Adam. 2007. Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring. Proceedings of the Royal Society B 274: 2563-2569.
Cockburn, A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273: 1375 - 1383.
Cooney et al. 2020. Ecology and allometry predict the evolution of avian developmental durations. Nature Communications 11: 2383.
Davies, N. B., R.M. Kilner, and D.G. Noble. 1998. Nestling cuckoos Cuculus canorus exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B 265 : 673–678.
de Framond, L., H. Brumm, W. I. Thompson, S. M. Drabing and C. D. Francis. 2022. The broken-wing display across birds and the conditions for its evolution. Proc. Roy. Soc. B 289: 20220058.
DeGregorio et al. 2014. Snake predation on North American bird nests: culprits,patterns and future directions. Journal of Avian Biology 45: 325–333.
del Hoyo, H.J., A.Elliott, and J.Sargatal (eds). 1997. Handbook of the Birds of the World, Vol.4. Sandgrouse to Cuckoos. Lynx Edicions, Barcelona.
Dial, Kenneth P. 2003. Evolution of avian locomotion: correlates of flight style, locomotor modules, nesting biology, body size, development, and the origin of flapping flight. Auk 120:941-952.
Ehrlich, P., D. Dobkin, and D. Wheye. 1988. The Birder's Handbook. Simon and Schuster, New York, NY.
Erickson, G. M. 2005. Assessing dinosaur growth patterns: a microscopic revolution. Trends in Ecology and Evolution 20: 677-684.Forbes, S. and D.W. Mock. 2000. A tale of two strategies: life-history aspects of family strife. Condor 102:23-34.
Gauthier-Clerc, M., Y. Le Maho, Y. Clerquin, S. Drault, & Y. Handrich. 2000. Ecophysiology: penguin fathers preserve food for their chicks. Nature 408:928-929.
Gill, F.B. 1995. Ornithology, second ed. W.H. Freeman and Co., New York, NY.
Godfray, H. C. J. 1991. Signalling of need by offspring to their parents. Nature 352: 328-330.
Gulson-Castillo et al. 2018. Coordinated misdirection: a probable anti-nest predation behavior widespread in Neotropical birds. Wilson Journal of Ornithology 130: 583-590.
Haff, T. M., and R. D. Magrath. 2011. Calling at a cost: elevated nestling calling attracts predators to active nests. Biology Letters 7: 493-495.
Heinsohn, R., N. E. Langmore, A. Cockburn, and H. Kokko. 2011. Adaptive secondary sex ratio adjustments via sex-specific infanticide in a bird. Current Biology 21: 1744-1747.
Kermott, L. H., L. S. Johnson, and M. S. Merkle. 1991. Experimental evidence for the function of mate replacement and infanticide by males in a north-temperate population of House Wrens. Condor 93: 630-636.
Kilner, R. M. 2001. A growth cost of begging in captive canary chicks. Proceedings of the National Academy of Science 98:11394-11398.
Lemel, J. 1989. Body mass dependent fledging order in the Great Tit. Auk 106: 490–492.
Long et al. 2022. Does ecology and life history predict parental cooperation in birds? A comparative analysis. Behavioral Ecology and Sociobiology 76: 92.
Lotem, A. 1998. Manipulative begging calls by parasitic cuckoo chicks: why should true offspring not do the same? Trends in Ecology and Evolution 13:342-343.
Martin et al. 2025. Parental effort in warming young: A neglected component of life history strategies influenced by nest structure, brood size, body mass and biparental care. Functional Ecology 39: 3299-3310.
Michaud, T. and M. Leonard. 2000. The role of development, parental behavior, and nestmate competition in fledging of nestling Tree Swallows. Auk 117:996-1002.
Nice, M.M. 1962. Development of behavior in precocial birds. Trans. Linn. Soc. N.Y. No. 6.
Olson, V. A., A. Liker, R. P. Freckleton, and T. Szekely. 2008. Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proceedings of the Royal Society B: 275: 301-307.
Østnes, J. E., B. M. Jenssen, and C. Becha. 2001. Growth and development of homeothermy in nestling European Shags. Auk 118: 983–995.
Owens, I.P.F. 2002. Male-only care and classical polyandry in birds: phylogeny, ecology and sex differences in mating opportunities. Phil. Trans. R. Soc. 357: 283-293.
Payne, R. B. 2006. Indigo Bunting (Passerina cyanea ). The Birds of North America Online (A. Poole, ed.). Ithaca: Cornell Laboratory of Ornithology; Retrieved from The Birds of North America Online database: http://bna.birds.cornell.edu/BNA/account/Indigo_Bunting/ .
Pettingill, O.S., Jr. 1985. Ornithology in laboratory and field, fifth ed. Academic Press, New York.
Price, E. R. and E. M. Dzialowski. 2018. Development of endothermy in birds: patterns and mechanisms. Journal of Comparative Physiology B 188: 373–391.
Radford, A. N. and A. R. Ridley. 2006. Recruitment calling: a novel form of extended parental care in an altricial species. Current Biology 16: 1700-1704.
Remeš, V. and T. E. Martin. 2002. Environmental influences on the evolution of growth and developmental rates in passerines. Evolution 56: 2505-2518.
Ricklefs, R.E. 1983. Avian postnatal development. Current Ornithology 1:1-32.
Rohwer, S. 1986. Selection for adoption versus infanticide by replacement 'mates' in birds. Current Ornithology 3: 353-395.
Ruaux, G. et al. 2020. The development of flight behaviours in birds. Proceedings of the Royal Society B 287: 20200668.
Roulin, A., M. Kolliker, and H. Richner. 2000. Barn Owl siblings vocally negotiate resources. Proc. Royal Soc. B 267:459-463.
Saino, N., P. Ninni, S. Calza, R. Martinelli, F. De Bernardi, & A.P. Møller. 2000. Better red than dead: carotenoid-based mouth coloration reveals infection in barn swallow nestlings. Proc. Royal Society B 267:57.
Sergio, F. and G. Bogliani. 2001. Nest Defense as Parental Care in the Northern Hobby (Falco subbuteo). Auk 118: 1047-1052.
Skutch, A.F. 1976. Parent birds and their young. University of Texas Press, Austin, TX.
Studer, A. and M. A. Crozariol. 2025. New breeding information on Brazilian birds. 3: Nyctibiidae, Caprimulgidae, Apodidae and Trochilidae. Bulletin of the British Ornithologists' Club 145: 193-272.
Szekely, T., G. H. Thomas, and I. C. Cuthill. 2006. Sexual conflict, ecology, and breeding systems in shorebirds. BioScience 56: 801–808.
Tanner, M. and H. Richner. 2008. Ultraviolet reflectance of plumage for parent-offspring communication in the Great Tit (Parus major). Behavioral Ecology 19: 369-373.
Trivers, R.L. 1974. Parent-offspring conflict. American Zoologist 14: 249–264.
Wang et al. 2023. Sex differences in avian parental care patterns vary across the breeding cycle. Nature Communications 14: 6980.
Welty, J.C. and L. Baptista. 1988. The life of birds, fourth ed. Saunders College Publishing, New York.
Wright, J., S. Markman, and S. M. Denney. 2006. Facultative adjustment of pre-fledging mass loss by nestling swifts preparing for flight. Proceedings of the Royal Society B 273: 1895-1900.
Zhou, Z. and F. Zhang. 2004. A Precocial Avian Embryo from the Lower Cretaceous of China. Science 306: 653.
Useful links:
Hatching Asynchrony and Brood Reduction
Parent-Chick Recognition in Colonial Nesters