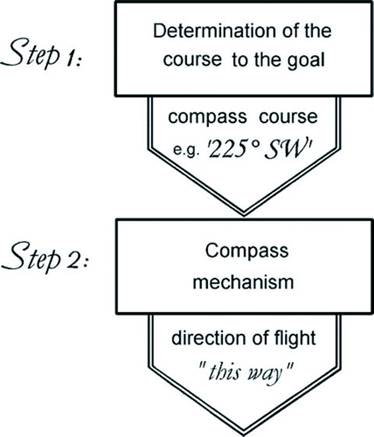
Figure 1. The two-step navigation system proposed by Kramer (1953, 1957)
(From: Wiltschko and Wiltschko 2009).
Avian Navigation and Orientation
(Any feedback on the material presented on this page, e.g., errors, omissions, or other comments would be appreciated.
Send your comments to Gary Ritchison at gary.ritchison@eku.edu).
Non-compass orientation | Star compass | Sun compass | Polarized light | Magnetic cues | True navigation | Long- and short-range navigation | Literature cited
Birds are often faced with the need to return to a particular location, such as a nest or roost site, a source of food or water, or, for migratory species, a breeding territory or wintering area. Such directed movement is called navigation or, more precisely, true navigation and involves the ability of a bird to locate its position, whether in a familiar or unfamiliar area, with respect to where it wants to go. Orientation, on the other hand, is more simply the ability to move in a given compass direction. True navigation, as first described by Kramer (1953, 1957), is a two-step process: (1) determining the correct direction of travel, and (2) being able to correctly identify that direction. In other words, birds must determine the direction that will take them toward their goal, just as we would when using a map, then, as we might using a compass, locate or identify that direction (Figure 1). Among birds, true navigation is typically accomplished only by ‘experienced’ birds - birds that have become familiar, on a smaller scale, with a local area or, on a larger, migratory scale, have successfully completed a migratory journey at least once.

Figure 1. The two-step navigation system proposed by Kramer (1953, 1957)
(From: Wiltschko and Wiltschko 2009).
For some species, young, naïve birds appear to be capable only of vector navigation, or the ability to maintain a particular orientation for a specified time or distance (Figure 2; Bingman and Cheng 2005). Differences between adult and juvenile birds have been revealed in experiments where they are captured during migration at the same location and then transported some distance from the normal migration route and released. Such experiments with European Starlings (Sturnus vulgaris; Perdeck 1958), Common Teal (Anas crecca; Wolff 1970), and White-crowned Sparrows (Zonotrichia leucophrys; Thorup et al. 2007) during fall migration have all demonstrated that adults typically compensate for the displacement and fly in the direction that will take them to their normal wintering grounds, whereas juveniles continue to fly in the direction they were moving prior to displacement (Fig. 3). These results indicate that, for these species, first-time migrants reach their wintering areas by an innate mechanism that influences the direction and distance of movement or, in other words, vector navigation.
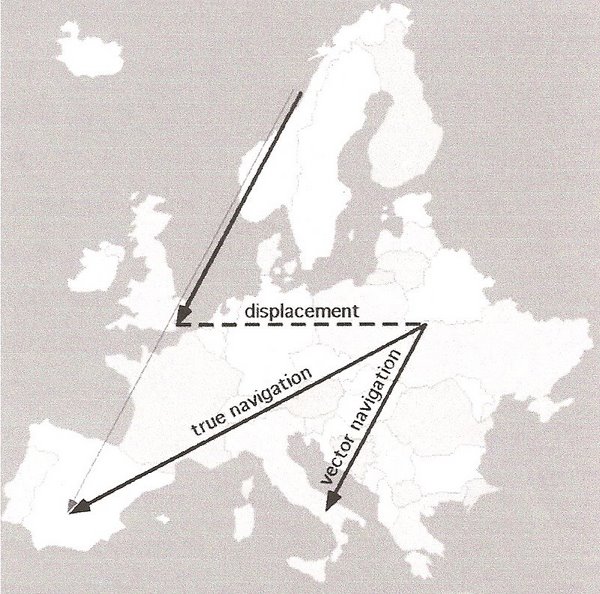
Figure 2. Example of the difference between true navigation and vector navigation
by a hypothetical songbird migrant in Europe
with a fall migration route from Norway to Spain (thin solid arrow). In this hypothetical
experiment, migrants en route are captured
and displaced (dashed arrow) from their traditional migratory path to a distant, unfamiliar site in eastern Europe and then released.
True navigation (the thick solid arrow to Spain) would require the ability to determine the ‘new’ location and adjust the
migratory
route to compensate for the displacement and still end up at the over-wintering site in Spain. Because vector navigation (the thick solid
arrow to Italy) is only the ability to continue moving in a particularly direction for a certain distance or time, there would be no
compensating to take into account the ‘new’ location. So, migration would continue along the some orientation and for the same distance,
but the hypothetical migrant would end up in Italy rather than Spain (From: Bingman and Cheng 2005).

Figure 3. Thirty White-crowned Sparrows (15 adults and 15 juveniles) were displaced 3700 km from Seattle,
Washington, USA, to Princeton, New Jersey, USA. The map on the left shows the displacement and breeding
area (green), wintering area (cyan) and normal migration route (blue). The circle to the right shows the directions with
the mean and confidence interval indicated where adults (blue) and juveniles (red) flew after being released in New Jersey.
Adults flew in the direction toward their normal wintering grounds, whereas juveniles continued in their normal
migration direction. (From: Thorup et al. 2007).
Results of other studies suggest that, in some species, juveniles may be able to compensate for displacement. Using captive birds, Åkesson et al. (2005) recorded the orientation of migratory White-crowned Sparrows after longitudinal displacement of distances ranging from 266–2862 km across high-Arctic North America and found that both adults and juveniles compensated for the displacement and altered their orientation in the manner that would take them back either to their normal wintering areas or to their normal migration route. Thorup and Rabøl (2007) re-analyzed the results of several displacement experiments and found that, in many cases, both adults and juveniles showed directional shifts after displacement.
Studies to date, then, indicate that, among migratory species, adults and, in some cases, juveniles are capable to true navigation. For juveniles, this ability must be innate. For species where adults are capable of true navigation and juveniles are not, learning takes place during the first migratory journal and birds acquire the information needed for true navigation. Regardless of whether innate or learned, true navigation requires the ability to discern one’s location with respect to the ultimate destination or goal (Thorup and Holland 2009). For a bird returning to a particular location in a known or familiar area like a nest in a breeding territory, visual landmarks recognized from previous visits can be used to determine its location and the correct direction of travel. However, navigation from an unfamiliar area requires some other mechanism for determining one’s location.
Navigation by humans is based on the latitude/longitude coordinate systems. True navigation by birds is likely based on a similar bi-coordinate, or grid-based, system, with cues varying along gradients that allow extrapolation of familiar gradients to unfamiliar areas (Thorup and Holland 2009; Fig. 4). What is not currently known with certainty is what those cues are. However, the cues considered most likely to be important in bird navigation are celestial, magnetic, and olfactory cues. Each of these same cues can also be used for orientation by birds and, before examining how birds might use them for navigation (to determine their location), an understanding of how they function is needed.
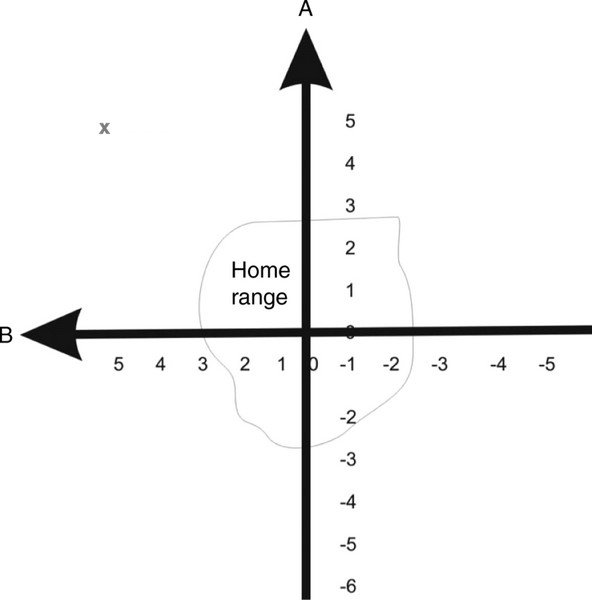
Fig. 4. With a bi-coordinate gradient map, birds learn the navigational cues that vary in strength in their territory or home range.
Birds then assume by extrapolation that these cues continue to vary in this way elsewhere. For long-distance migrants, these cues
would need to vary consistently on a continental or global scale. As an example of how this mechanism works, if a bird is located
outside of its range at point X (A5, B5), then detected cues are greater or stronger than encountered previously (because cues
are increasing in strength as indicated by the direction of arrows A and B). A bird then knows it is north and west of its home range
and must fly southeast to return (From: Thorup and Holland 2009).
In some cases, birds orient their flight relative to landmarks or topographic features. For example, during migration, migrants may orient their flight using coastlines, mountain ridges, or rivers. In Europe, many birds migrating south that encounter the Alps turn southwest and fly parallel to the mountains, with fewer flying through passes between the mountains (Liechti et al. 1996, Bruderer and Liechti 1999). Similar behavior has been reported for birds migrating along the northern Appalachian mountains (Williams et al. 2001). Some nocturnal migrants in eastern New York appear to travel along routes parallel to the Hudson River (Bingman et al. 1982), and diurnally migrating raptors are well known for migrating along prominent topographic features such as coastlines and mountain ridges (Figure 5; Bildstein 2006).
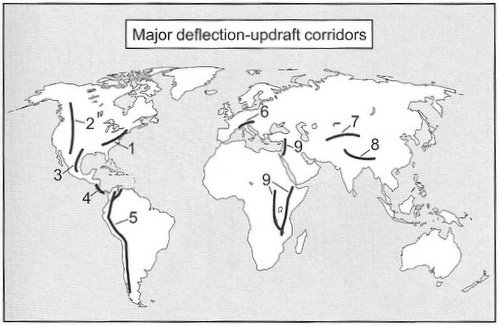
Figure 5. Mountain ranges and ridges that generate updrafts used by migrating raptors. (1) Appalachian Mountains,
(2) Rocky Mountains, (3) Sierra Madre Oriental, (4) Talamanca Mountains, (5) Andes, (6) Alps, (7) Tien Shan and Hindu Kush,
(8) southern Himalayas, and (9) ridges along the Great Rift Valley (From: Bildstein 2006).
Compass orientation - star compass
In a series of classic experiments, Emlen (1967a, b, 1969, 1970) showed that Indigo Buntings (Passerina cyanea) use the stars for orientation during migration. By manipulating the location and movements of stars and constellations in a planetarium, Emlen (1967b) found that buntings derived directional information from the pattern of constellations relative to each other and to the celestial pole. Specifically, young buntings learned the location of ‘north’ by observing the rotation of constellations around the celestial pole. In these planetarium experiments, Emlen (1966) determined the direction that buntings were attempting to fly, or their orientation, using funnels with ink pads at the bottom and lined with blotting paper (Emlen funnels; Fig. 6). Each time a bunting hopped upward (and exhibiting nocturnal restlessness, or zugunruhe, they did so frequently), ink on the feet and plumage was left on the blotting paper, leaving a record of the direction the bunting was attempting to travel (Fig. 6).
Star-compass orientation has been demonstrated in several species of night-migrating songbirds, including Bobolinks (Beason 1987), Savannah Sparrows (Able and Able 1996), and Garden Warblers (Sylvia borin; Weindler et al. 1997). As first reported by Emlen (1967a, b), birds that use stellar cues for orientation do not do so innately, but must learn to use this ‘compass’ by observation of stellar rotation. Experiments have also revealed that birds do not rely on specific stars like the north star (Polaris), but obtain directional information from the position of stars and constellations relative to each other and to the celestial pole (Wiltschko and Wiltschko 2009). This means that songbirds in the southern hemisphere (where there is no bright star directly or nearly directly above the south pole) are also able to use star-compass orientation.
Although it seems likely that most, if not all, night-migrating songbirds (Passeriformes) are capable of star-compass orientation, little is known about the possible use of stellar cues for orientation by other taxa of birds. Of course, an ability to orient using stellar cues is unneeded for birds that migrate during the day, such as raptors and waterfowl. However, some non-songbirds, such as some owls, do migrate at night and studies examining cues used for orientation by such species are needed. In addition, nothing is currently known about the possible ability of birds that are normally active at night, such as owls and caprimulgids, to orient using stellar cues.
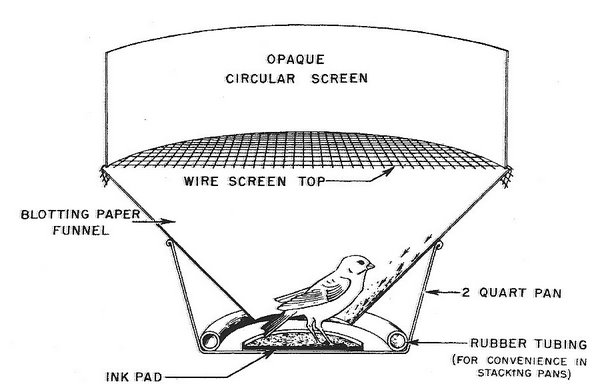

Fig. 6. Top, an ‘Emlen funnel.’ Birds could view the planetarium ‘sky’ through the opaque circular screen.
Bottom, an example of a ‘footprint’ record (left) and a resulting vector diagram (right) for quantifying the footprint records (From: Emlen 1966).
A Sharp-tailed Sandpiper in an orientation cage (i.e., an "Emlen funnel"). The bottom of the cage is lined with Tipp-ex paper,
with the bird leaving scratchmarks when trying to fly off. These marks indicate the direction the bird is trying to fly.
Compass orientation - sun compass
The use of the sun for orientation by birds was first demonstrated by Kramer (1950). He used a test apparatus where mirrors altered the apparent position of the sun and found that European Starlings altered their directional preference accordingly. The avian sun compass is based on the sun’s azimuth (the point on the horizon directly below the sun), with the sun’s altitude being irrelevant. Because the sun moves across the sky during the day, the use of a sun compass requires an internal clock because it is the sun’s azimuth at a particular time of day that provides directional information.
The importance of a bird’s internal clock for correctly using the sun compass can be demonstrated by ‘shifting’ the clock. For example, Schmidt-Koenig (1958) found that the bearings of Rock Pigeons that had been kept for several days in a room where the ‘artificial’ photoperiod was shifted by six hours either forward or backward differed from those of non-shifted pigeons by about 90 degrees. How can this be explained? As an example, assume two pigeons, one non-shifted and one shifted six hours forward, are released at noon (12:00) on a clear day and both attempt to return to a home loft located 10 km to the south. Upon release, both pigeons see the sun, but the ‘clock-shifted’ pigeon mistakenly interprets the ‘noon sun’ as the 6:00 p.m. (18:00) sun. So, the non-shifted pigeon knowing the ‘noon sun’ is located to the south will, correctly, fly south. However, the clock-shifted pigeon incorrectly interprets the ‘noon sun’ as the ‘evening sun’ to the west and, intending to fly south, flies east instead.
Birds using the sun for orientation must compensate for the sun’s movement, a task made more difficult by variation in the speed of movement during the day. For example, shortly after sunrise and before sunset, the sun’s altitude changes rapidly, but the azimuth changes slowly. At mid-day, in contrast, the altitude changes slowly, but the azimuth changes rapidly. In addition, the rate of change in the sun’s azimuth varies with latitude and season. Despite this apparently complexity, birds that use the sun compass appear to have a precise understanding of how the sun’s azimuth changes not only throughout a day, but with season and latitude (Wiltschko et al. 1998, Duff et al. 1998, Wiltschko et al. 2000).
Birds must learn to use the sun compass and current evidence indicates that the area of the brain most important in this process is the left hippocampus (Gagliardo et al. 2005). Few investigators have examined the process by which birds learn to use the sun compass. Studies of young homing pigeons indicate that they are first able to use the sun compass when about 12 weeks old. However, the specific time of development of the sun compass depends on flying experience, with learning taking place after a pigeon is confronted with the need to orient during flights around its home loft (Wiltschko and Wiltschko 1981).
Compass orientation – polarized light
Gas and water molecules in the atmosphere scatter light from the sun in all directions, an effect that is responsible for blue skies and a phenomenon called atmospheric polarization. When a photon from the sun strikes a gas molecule, the electric field from the photon induces a vibration and subsequent re-radiation of polarized light from the molecule (Figure 7). Light scattering off atoms and molecules in the atmosphere is unpolarized if the light keeps traveling in the same direction and is linearly polarized if at scatters in a direction perpendicular (either vertically or horizontally) to the way it was traveling. So sunlight coming directly toward you is unpolarized. Light is more polarized in directions perpendicular to the sun's rays so, at noon, polarization would be most apparent along the horizon. However, at sunset, polarized light forms an image like a large bow-tie -- located overhead at sunset -- pointing north and south. Importantly, polarization patterns are apparent even when skies are cloudy (Figure 8).
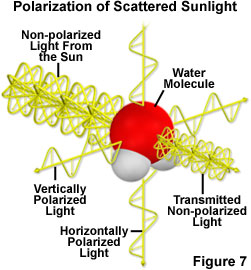
Fig. 7. Light striking atoms and molecules in the atmosphere is unpolarized if it keeps traveling in the same
direction and is
polarized if it scatters in a perpendicular direction (either vertically or horizontally)
(From: http://www.microscopyu.com/articles/polarized/polarizedlightintro.html).
Clear sky 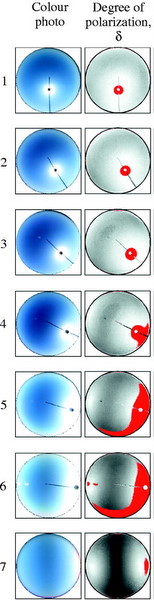 |
Cloudy sky |
Figure 8. Degree of polarization measured by full-sky imaging polarimetry over the entire clear sky (left)
and an entire cloudy sky (right) during the period from 13:00 (# 1) through 19:00 (# 7, sunset) at a latitude
of 33°52' N (near Kriz, Tunisia). The position of the sun is indicated by the black dot. Notice that, at 13:00,
polarization is most apparent along the horizon. However, at sunset, polarized light forms an image like a large bow-tie --
located overhead at sunset -- pointing north and south (From: Pomozi et al. 2001).
Many invertebrates and vertebrates can perceive polarized light and use that ability in activities such as foraging and orientation. Although it remains to be determined how many species of birds can detect polarized light and, for those that can, the mechanism involved (Greenwood et al. 2003), experiments indicate that several species of migratory birds (but apparently not all; Wiltschko et al. 2008) are apparently able to perceive polarized light and use it as a cue for orientation. Specifically, investigators have provided experimental evidence that migratory Savannah Sparrows (Passerculus sandwichensis; Muheim et al. 2006), Swainson’s (Catharus ustulatus) and Gray-cheeked (C. minimus) thrushes (Cochran et al. 2004), and White-throated Sparrows (Zonotrichia albicollis; Muheim et al. 2009) use polarized light cues at sunrise or sunset to calibrate their magnetic compass. Because the relationship between magnetic and geographic north changes with location, birds need to calibrate the different compass systems with respect to each other on a regular basis to prevent navigational errors (Muheim et al. 2006, 2007).
Neural correlates of a magnetic sense -- Wu and Dickman (2012) have shown that single vestibular brainstem neurons encode the direction, intensity, and polarity of an applied magnetic field in pigeons; consistent with a ferrimagnetic particle receptor, as opposed to a radial-pair cryptochrome mechanism. These findings demonstrate that MR neurons (vestibular brainstem neurons) are most sensitive within an intensity range that is naturally produced by the Earth’s magnetic field, a necessary condition for a magnetoreception system to be useful in the derivation of geopositional information. However, the Earth’s magnetic field varies over time (e.g., a 35% decrease in strength over the last 2000 years), so it would seem likely that magnetoreception systems adapt through evolution and/or developmental plasticity to the slowly changing fields in order to maximize magnetic sense perception. It is likely that MR neurons receive magnetic information from the inner ear lagena; however signals from the beak and/or retina are also possible. As MR neurons are located in the vestibular nuclei, multimodal integration of magnetic and linear acceleration cues could provide geomagnetic information relative to the fixed constant of gravity. If so, magnetoreception neural constructs would remain stable in a space-fixed reference frame, regardless of head position. Wu and Dickman suggest that MR cells encode a geomagnetic vector that could be used by the neural population to computationally derive the bird’s position and directional heading. The geomagnetic vector elevation component could provide the bird’s latitude, the vector azimuth component could be used as a magnetic compass to provide heading direction, and the vector magnitude could provide spatial position cues through local variations in intensity relative to a learned internal model of geomagnetic space. How MR cell information is used for orientation and navigation remains to be discovered, but these findings demonstrate a direct neural substrate underlying a magnetic sense in the avian brain.
Links:
Pigeons may 'hear' magnetic fields
Neurons in a pigeon's brain respond to magnetic fields
Pigeon brain's global positioning system located
Compass orientation - magnetic cues
The hypothesis that migrating birds could use the earth’s magnetic field for orientation was first proposed in 1859 (von Middendorff 1859), and experimental evidence to support that hypothesis was first provided in 1968 in a study of European Robins (Wiltschko 1968). Since then, the use of a magnetic compass has been demonstrated in several species of migrants (19 passerines and one shorebird, Sanderling; Wiltschko and Wiltschko 2009, Huttunen 2009) as well as two non-migratory species (Zebra Finch, Taeniopygia guttata, Voss et al. 2007; Domestic Chicken, Gallus gallus, Freire et al. 2005). Because the lineages leading to present-day gallinaceous birds (order Galliformes) and songbirds (order Passeriformes) likely separated as early as the Cretaceous period (Cooper and Penny 1997), the magnetic compass of birds likely developed before then and may even have developed in the common ancestors of all modern birds (Wiltschko et al. 2007).
The earth’s magnetic field, caused by electrical current generated by the rotating molten core, is slightly tilted relative to the spin axis so the two poles (magnetic north and south) are located several hundred kilometers from the geographic poles. Field lines leave the earth at the southern magnetic pole, curve around (forming the magnetosphere), then re-enter the earth and the northern magnetic pole (Figure 9). Near the equator, magnetic field lines run parallel to the earth’s surface and the inclination of the field lines become progressively steeper moving from the equator toward the magnetic poles where the inclination angle is 90 degrees.
The compasses we use simply point to magnetic poles (the north magnetic pole in the northern hemisphere and the south magnetic pole in the southern hemisphere), or the points where the earth’s magnetic field is vertical. In contrast, the avian magnetic compass is an inclination compass. That is, birds use the inclination of magnetic field lines relative to the earth’s surface (Fig. 9). An inclination compass does not distinguish between north and south, but between the direction of the poles, where field lines are inclined increasingly steeper, and the direction of the equator, where the inclination in increasingly shallow. For birds, the north and south magnetic poles cannot be distinguished because their ‘compass’ only distinguishes between the direction of a pole and the direction of the equator. Birds that cross the equator, then, must somehow switch from a ‘move toward the equator’ strategy to a ‘move toward the pole’ strategy. Experience with the horizontal magnetic field found at the magnetic equator appears to trigger this switch (Beason 1992).

Fig. 9. Representation of the earth’s magnetic field showing how field lines (represented by arrows)
intersect the earth’s surface, and how inclination angle varies with latitude.
The avian inclination compass differs from human compasses in another important and surprising way – it is light dependent. In other words, birds actually ‘see’ magnetic field lines (in a way, of course, that we can only imagine). Birds ‘see’ magnetic field lines because the receptors are located in the retina of the avian eye. Specifically, the magnetic sense may be based on a light-photoreceptor protein called cryptochrome found in the retina of the avian eye. Cryptochrome appears to be located primarily in ganglion cells (Fig. 10), but is also found in photoreceptors (cones) and in other cells in the inner layer of the retina (Mouritsen et al. 2004). Although questions remain concerning the mechanism, one possibility is that a t dusk and at night, dim light, predominantly in the blue-green spectral range, excites the photoreceptor cryptochrome molecules in a bird’s retina. The light excitation initiates a reaction involving the formation of a pair of radicals (molecules with a single, unpaired electron) and electron-transfer within cryptochrome molecules, and magnetic fields can influence this pathway by affecting the spin or rotation of the electrons. Because of the rounded shape of the retina, the magnetic field affects the cryptochrome molecules differently in different parts of the retina.

Fig. 10. Garden Warbler (Sylvia borin; above) and cross-section through the retina of a Garden Warbler showing ganglion cells
(at the bottom of the image) where cryptochromes are located. The rods and cones would be located at the top of the image
(From: Miller 2004, Mouritsen et al. 2004).
As a result, the magnetic field is translated into a visual pattern transmitted to the brain from the retina (Mouritsen and Ritz 2005). Assuming this reaction occurs best when the magnetic field is parallel to cryptochrome molecules, a bird looking in different directions relative to the magnetic field might ‘see’ visual patterns that look something like that illustrated in Figures 11 and 12, and using this information, be able to determine its direction of movement relative to a magnetic pole (north or south) and the magnetic equator. In fact, migratory birds relying on their magnetic compass are known to look in several directions (head scans), apparently to determine the appropriate direction, before initiating activity (Mouritsen et al. 2004).

Fig. 11. A bird’s-eye view of the geomagnetic field as seen when looking in different directions. In this example, the
geomagnetic field (arrow) has an inclination of 68 degrees (From: Ritz et al. 2000).
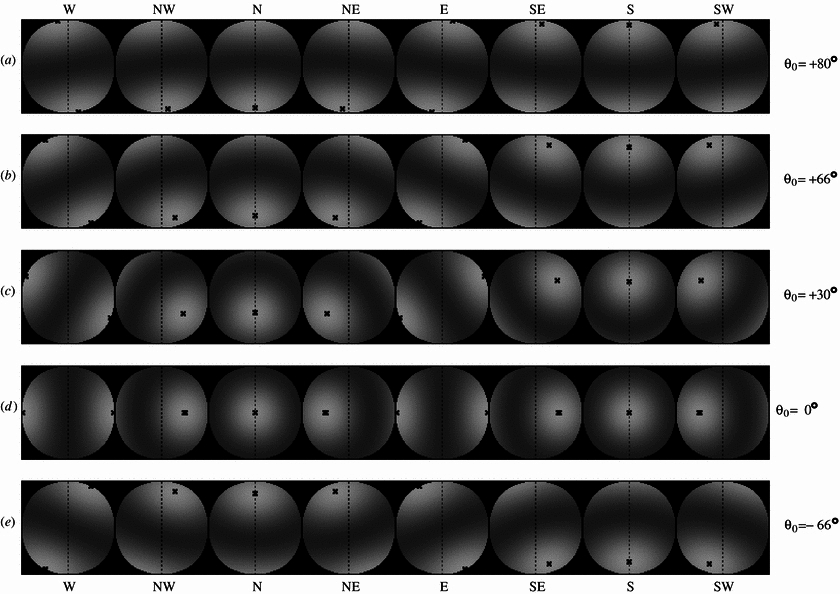
Figure 12. Another bird-eye view of the magnetic field for a bird changing its viewing direction clockwise in 45° increments in magnetic
fields of
different inclinations. For easier recognition, the crosses mark the position of the brightest spot in the signal modulation patterns. For
each inclination,
the eight panels represent a full 360° sweep, showing all cardinal directions, from west (left panel) to southwest (right panel).
Inclination angles are positive for the northern hemisphere with the magnetic field pointing downward. (a) + 80° inclination, e.g. in northern
Canada, (b) + 66° inclination, e.g. in central Europe, (c) + 30° inclination, e.g. in the Sahara desert, (d) 0° inclination, at the equator,
(e) –66° inclination, e.g. in southern Australia (From: Wang et al. 2006; http://iopscience.iop.org/1478-3975/3/3/007/fulltext).
When birds are using magnetic cues for orientation, an area in the forebrain called cluster N becomes active. In contrast, neuronal activity in cluster N does not increase in non-migratory birds during the night, and activity ceases when a bird’s eyes are covered, indicating that there is a direct neuronal connection between the retina and cluster N. This connection, plus the location of cluster N adjacent to and part of a visual area of the brain called the visual Wulst, indicates that migratory birds use their visual system to ‘see’ the geomagnetic field (Heyers et al. 2007). In support of this hypothesis, Zapka et al. (2009) showed that European Robins with lesions of cluster N were no longer able to orient using magnetic fields, but were still able to orient correctly using their star compass and sun compass (Figure 13).

Figure 13. a, European Robins with a damaged cluster N that were tested in a planetarium simulating the local starry sky
(STN, star north)
oriented in the typical north-northeast spring migratory direction). b, Birds with cluster N damage could not orient using their magnetic compass
(MPW, magnetic pole). c, Birds with cluster N damage could also orient during sunset using their sun compass (From: Zapka et al. 2009).
| Is the light-dependent magnetic compass of birds lateralized? -- Night-migratory birds can use a magnetic compass to find their way during long-distance migration, and most recent evidence indicates that the magnetic compass of night-migratory birds is based on light-dependent, radical-pair reactions in the birds’ eyes. The results of some studies suggest that this light-dependent magnetic compass is located only in the right eye of birds (Wiltschko et al. 2002, 2003). However, Hein et al. (2009) found that night-migrating Garden Warblers (Sylvia borin) were able to perform magnetic compass orientation with both eyes open, with only the left eye open, and with only the right eye open. Studies of other species of birds have demonstrated that light photoreceptor molecules (cryptochromes) are found in both eyes and the area of brain thought to be important in magnetic compass orientation (cluster N) is active in both hemispheres of the brain. Clearly, additional studies of other species are needed to better understand the apparent species differences in how birds obtain magnetic compass information. |
Birds may be able to use any or all of the compass mechanisms just described, but a compass is useful for reaching a specific location or goal, such as a nest site, food cache, wintering area or breeding territory, only if a bird also knows its current location. Once a bird determines its current spatial location relative to some distant goal (typically called ‘map’ information), it can then choose the appropriate direction of travel.
With the exception of birds transported to previously unknown locations so investigators can study their navigational abilities, birds can acquire map information about their surroundings. In areas a bird is familiar with, such as a breeding territory, map information can be based on visual landmarks as well as local magnetic, auditory, and olfactory cues. Several species of birds, including homing pigeons and birds that regularly store and retrieve food from caches, are known to use of landmarks to locate specific, previously visited sites. Among food-storing species such as jays, Clark’s Nutcrackers (Nucifraga columbiana), and parids, experimental studies have revealed that memory of the spatial locations of food caches is the primary means of locating caches (Kamil and Gould 2008). Homing pigeons use other cues to identify the general location of their home loft or other goal, but the final step in the process of homing appears to involve the visual recognition of landmarks (Gagliardo et al. 2007).
Birds could also potentially navigate using a process called path integration (sometimes referred to as dead reckoning). Path integration is a navigational, or homing, strategy used by many animals, ranging from arthropods to mammals. Using path integration, an animal is able to return to a specific location after travelling to any point some distance from it, even if the path taken is circuitous, by using information collected during the journey to determine a direct (straight-line) route back. Using path integration, an animal determines its position and the positions of other objects in the environment by integrating the distance and directions travelled during a journey. Distance and direction information can potentially be obtained from a number of sources, including proprioceptive cues, vestibular or somatosensory cues, and solar and magnetic cues. Among birds, Wiltschko and Wiltschko (1998) suggested that young pigeons use path integration (or, using their terminology, route reversal) when initially learning about their environment (until they are about three months old). However, Wallraff (2000: F34) concluded that the hypothesis that “pigeons develop a very sophisticated path integration mechanism for use within only a few weeks after which they forget it lacks both plausibility and experimental support.” Beyond homing pigeons, little is known about the use of path integration by birds. However, because the likelihood of errors increases with increasing distance (Able 2000), path integration, if used by birds, is likely of greater importance for short-distance navigation.
Landmarks may be useful for short-range homing (returning to a familiar area or site), but how do birds located at greater distances from a target destination determine where they are located? The short answer is that we do not know. As noted earlier, a grid-based navigational system seems most likely because ‘map-based’ cues (those that a bird can memorize and use to navigate) are too local to be useful over long distances (e.g., visual landmarks). Cues other than visual landmarks, such as atmospheric odors and infrasounds (very low frequency sounds generated by ocean waves and mountain winds), could potentially be used as features of a ‘map-based’ navigational system.
The possible importance of olfactory cues in bird navigation has only been examined in two species. Studies of homing pigeons have revealed that olfactory cues may be important for successful homing, with experienced pigeons hypothesized to use an ‘olfactory map’ (based on the spatial distribution of natural odor sources) to determine their position and navigate (Wallraff 2004). However, Jorge et al. (2009) reported evidence that olfactory cues do not provide homing pigeons with navigational cues, but, rather, detection of ‘non-home’ odors simply activates other cues used for navigation. Holland et al. (2009), by treating some individuals with zinc sulphate to produce anosmia, found that olfaction may play some role in determining the direction of migration by adult Gray Catbirds (Dumetella carolinensis), but how catbirds might use olfactory information relative to other cues remains to be determined. Given the few species studied, the conflicting results of studies with the most-studied species (homing pigeons), and the improbability of navigation over distances beyond a few hundred kilometers using airborne olfactory cues (Bingman and Cheng 2005), long-range navigation using olfactory ‘maps’ currently seems unlikely for most birds.
One possible exception, however, may be the tube-nosed seabirds (order Procellariiformes). These seabirds have large olfactory bulbs and a well-developed sense of smell. Experiments have revealed, for example, that Antarctic Prions (Pachyptila desolata) can detect dimethyl sulfide (DMS) at very low concentrations (Nevitt and Bonadonna 2005). In the marine environment, DMS is known to be associated with the abundance of phytoplankton that is in turn associated with predictable oceanic features (Nevitt 2000; Figure 14). Although clear-cut evidence is lacking, olfactory cues, such as DMS, might be used to generate olfactory maps that could be used for navigation by tube-nosed seabirds.

Figure 14. Dimethyl sulfide emissions increase over ocean waters where phytoplankton are plentiful
(e.g., above upwellings that bring nutrients into the water column) and are being actively grazed by zooplankton.
Such emissions are often predictably present at certain locations and these locations may provide tube-nosed seabirds
with
navigational guideposts (From: Nevitt 2008).
Infrasounds are sound waves with frequencies below about 15 to 20 Hz, the lower threshold of sound detection by humans. Higher-frequency sounds tend to be attenuated by the atmosphere, but infrasounds can travel many thousands of kilometers. The use of an ‘infrasound map’ for navigation would require persistent sources of infrasounds that birds could hear. Persistent infrasounds are generated by mountain winds and ocean waves (Hagstrum 2000), and laboratory tests indicate that some birds, including pigeons (Kreithen and Quine 1979) and Helmeted Guineafowl (Numida meleagris; Theurich et al. 1984), can detect infrasounds. However, other species of birds are apparently unable to detect infrasounds (Beason 2004) and, for the vast majority of bird species, the ability to detect infrasounds has not been tested. In addition to questions concerning how many species can detect ultrasounds, Bingman and Cheng (2005) also point out that the assumed primary sources of infrasounds for birds, mountain winds and ocean waves, are not point sources. In other words, a bird capable of hearing infrasounds and migrating, for example, through the central United States would potentially hear those sounds being emitted from oceans on the east, west, and Gulf coasts and from the entire length of the Rocky and Appalachian mountain chains. It seems unlikely that birds could generate an ‘infrasound map’ using similar sounds simultaneously emanating from such widely distributed sources.
Humans navigate using a bi-coordinate system. Determine your latitude and longitude (easily accomplished with a GPS unit) and you know exactly where you are located. Based on our knowledge to date, it seems likely that avian navigation is also based on a bi-coordinate system (but it could very well be multi-coordinate; Thorup and Holland 2009). If so, navigating birds need some way to discern two coordinates (likely corresponding to our latitude and longitude). Birds can potentially determine their location along one coordinate (latitude) using two magnetic cues: inclination and, with experience, intensity (Gould 1998). Inclination provides latitudinal information because the inclination of the earth’s magnetic field, as noted previously, varies predictable with latitude (Figure 15), and many birds can ‘see’ this inclination. Although exhibiting more variability than inclination, the intensity of earth’s magnetic field also, in most areas, exhibits gradients that vary with latitude (Figure 16). To be used useful for navigation, birds must be able to ‘sense’ the intensity of the magnetic field and many birds are known to have special receptors that allow them to do so.
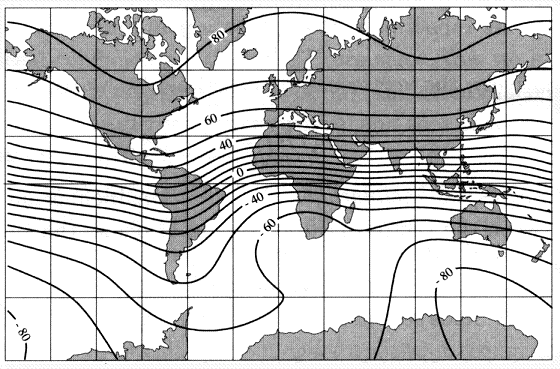
Figure 15. Isoclinic map showing latitudal variation in the inclination (in degrees) of earth’s magnetic field
(Source: http://geophysics.ou.edu/solid_earth/notes/mag_earth/earth.htm).
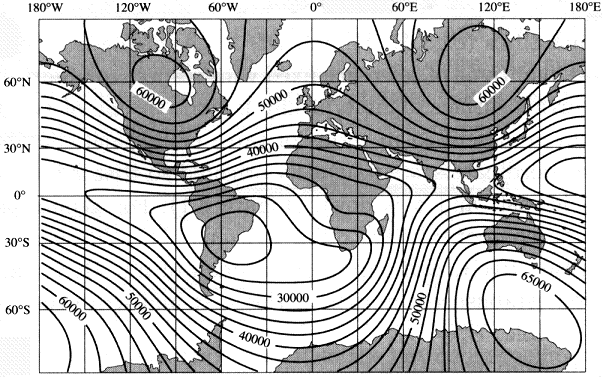
Figure 16. Isodynamic map should total intensity or strength of earth’s magnetic field, with higher values indicating greater intensity
(Source: http://geophysics.ou.edu/solid_earth/notes/mag_earth/earth.htm).
Receptors that ‘sense’ magnetic fields have been reported in several species of birds, including homing pigeons, European Robins, domestic chickens, and Silvereyes. These species, and almost certainly many others, have magnetic iron-oxide particles particles in their heads (and, specifically, in certain neurons), primarily in the upper beak. Electrophysiological recordings from associated nerves (Semm and Beason 1990) and the results of several behavioral studies (e.g., Mora et al. 2004, Stapput et al. 2008) indicate that these iron-based receptors provide birds with information about magnetic field intensity and direction.
The iron oxide particles in the beak of birds come in different forms (Figure 17). Tiny flattened structures called platelets (consisting of a ferromagnetic mineral called maghemite) form straight chains that are aligned along the dendrites of a neuron. The chain of maghemite platelets attracts a cluster of magnetite particles that are connected to the nerve cell membrane. A chain of maghemite platelets with a magnetite cluster function as a single magnetoreceptor unit, and there are many of these units in the beak that react to changes in the external magnetic field ( Solov’yov and Greiner 2009 ).
Each dendrite senses only one direction of the magnetic field (Fleissner et al. 2007), but there are several dendrites with different alignments so birds can monitor different directions of the magnetic field. Changes in the direction and strength of the magnetic field cause ion channels associated with the magnetoreceptors to open or close (Figure 18), and the resulting nervous impulses provide birds with information about the magnetic field. Because of the differing alignments of the dendrites, these receptors appear to act as a three-axis magnetometer, allowing birds to sense both magnetic field duration and the strength or intensity of the magnetic field (Fleissner et al. 2007, Dennis et al. 2007).
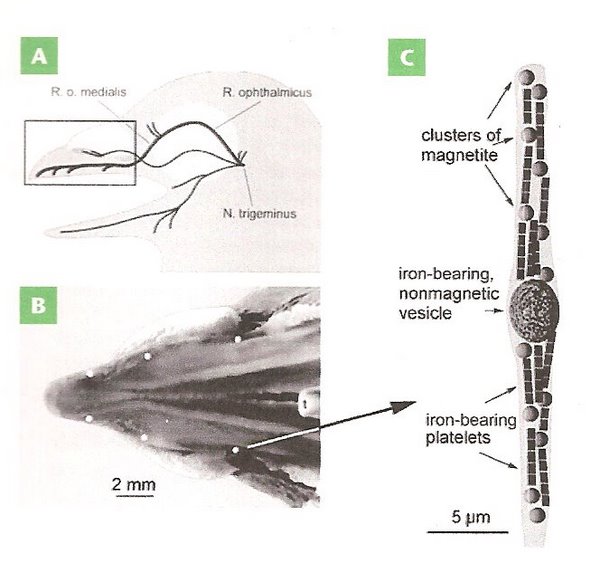
Figure 17. Magnetoreceptors in the bill of a homing pigeon. (A) Pigeon head showing location of nerves in the upper beak thought to
play a role in magnetoreception. (B) The upper beaked viewed from below showing the location (the six white spots) of iron oxide particles.
(C) Diagram of the end of a neuron (dendrite) containing a non-magnetic vesicle, several clusters of magnetite crystals, and chains of
iron-bearing platelets (From: Pósafi and Dunin-Borkowski 2009).

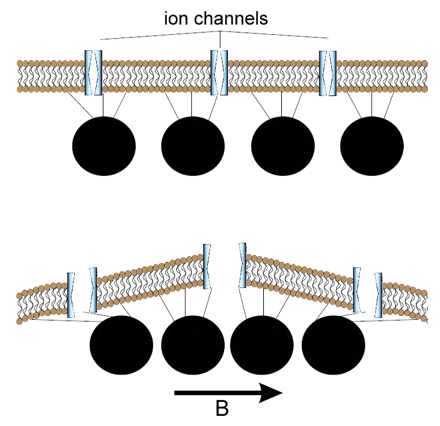
Figure 18. Possible magnetic sensors based on magnetite particles. Top, A chain of magnetite crystals is attached by protein
strands to ion channels in a nerve cell membrane. Ion channels open, causing nervous impulses, and close in response to the magnetic
field (indicated by the arrow. B). Below, Vesicles (black circles) filled with magnetite particles are attached to a membrane by
protein strands and, in response to the external magnetic field (arrow, B) the vesicles move towards each other, deforming the membrane,
opening the ion channels, and triggering a nervous impulse (From: Pósfai and Dunin-Borkowski 2009).
If their magnetic receptors provide information about the inclination (the light-based receptor), strength, and direction of magnetic fields, how might birds use that information to generate grid-based navigational maps? A grid-based navigational map requires that birds determine both their latitudinal and longitudinal position. Latitude can be determined using the inclination and, perhaps, the strength of the magnetic field. In some locations, lines of equal inclination and lines of equal strength are nearly parallel and oriented in an east-west direction, potentially allowing birds to determine latitude using either (or both) cue(s). However, in other locations, lines of equal inclination and lines of equal strength are not parallel and lines of equal strength are not oriented in an east-west direction. In such areas, the two sources of magnetic information might provide birds with information about both their latitude and longitude or, in other words, a bi-coordinate magnetic map.
One area where the lines of equal magnetic inclination and lines of equal magnetic strength or intensity vary in direction to form a grid-like pattern is northwestern Russia. Chernetsov et al. (2008) examined the navigational abilities of adult European Reed Warblers (Acrocephalus scirpaceus) during spring migration by displacing them about 1000 km to the east (Figure 19). The warblers corrected for the displacement by shifting their orientation from the northeast at the capture site (that would take them to their breeding ground) to the northwest after the displacement (Figure below). Such results indicate that the warblers were somehow able to determine that a longitudinal shift had occurred and were able to correctly orient in the direction that would take them to their breeding areas. Because lines of equal inclination and strength form a grid-like pattern in the study area, the warblers may have used those cues to accurately determine their position and then orient in the direction that would take them to their breeding areas.
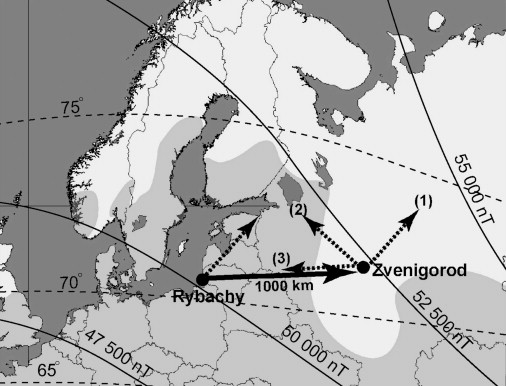
Fig. 19. Isolines of magnetic intensity (solid thin lines) and inclination (dashed lines) relative to capture (Rybachy, Kaliningrad region)
and displacement (Zvenigorod, Moscow region) sites and the breeding range of Eurasian Reed Warblers in the region (shaded light gray).
Solid arrow shows the displacement direction. The broken arrow at the capture site shows the mean migratory direction, and the broken
arrows at the displacement sites show the authors' working hypotheses: (1) no compensation, (2) compensation toward the breeding destinations,
and (3) compensation toward the capture site (From: Chernetsov et al. 2008).
A number of studies have revealed that birds have an innate ability to use their inclination compass; no experience is needed. However, using variation in magnetic strength to navigate requires experience because lines of equal magnetic strength vary in their orientation in different areas and, therefore, birds must learn the pattern of variation in the areas they occur. One possible illustration of this comes from the results of a study of White-crowned Sparrows (Thorup et al. 2007; see Figure 3 above). Adult White-crowned Sparrows displaced 3700 km from the west to the east coast of the United States were able to determine their ‘new’ longitudinal position and correctly orient in the direction that would take them to their wintering areas; juveniles incorrectly continued to orient as if they were still on the west coast. One explanation for such results is that the adult sparrows had acquired the needed magnetic information during previous migratory journeys, whereas juveniles had not.
In some areas, such as the United States (Figure 20), the lines of equal magnetic inclination and field strength are parallel, or very nearly so, and do not form a grid-like pattern and, therefore, may not, in combination, be useful for navigation.
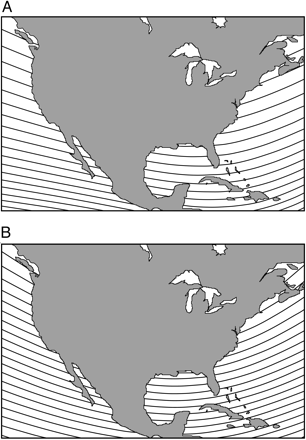
Figure 20. Isolines of magnetic field elements in North America. ( A) Isoclinics (isolines of
magnetic field inclination). Adjacent isoclinics represent differences in inclination of 2°. ( B) Isodynamics
(isolines of total field intensity). Adjacent isolines represent differences in intensity of 1,000 nT (From: Lohmann et al. 2008).
As an alternative, birds might use some combination of other magnetic cues for navigation, including inclination, total intensity, gradients of intensity, horizontal field intensity, vertical field intensity, declination (difference between true north and magnetic north; Figure 21), or, perhaps, even polarity (Solov’yov and Greiner 2009b). For example, some investigators have noted that magnetic intensity exhibits gradients (i.e., 'lines' between and through areas of different magnetic intensity; see Figure 22 below) between the poles and the magnetic equator (Walker 1998), and may provide birds with something like ‘magnetic latitude’ (Wiltschko et al. 2006).
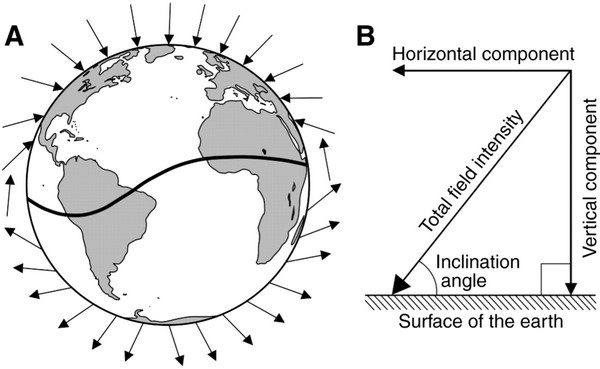
Figure 21. (A) Diagram of the Earth's magnetic field showing how the inclination angle varies with latitude. At the magnetic equator
(the curving line across the Earth), field lines are parallel to the Earth's surface. The field lines become progressively steeper as
one travels north toward the magnetic pole, where the field lines are directed straight down into the Earth and the inclination angle is
90°. (B) The four elements of Earth’s geomagnetic field. The field present at each location on Earth can be described in terms of
a total field intensity and an inclination angle. Total intensity has two vector components: horizontal field intensity and vertical
field intensity (From: Lohmann et al. 2008). Horizontal field intensity is greatest at the magnetic equator (where horizontal
field intensity equals total field intensity) and is lowest at the magnetic poles (where horizontal field intensity is zero);
the reverse is true for vertical field intensity.
Few investigators have examined the possibility that birds might use these gradients for navigational purposes. However, Dennis et al. (2007) released homing pigeons in areas in and around a natural magnetic anomaly (where the Earth’s magnetic field is spatially distorted and caused by differences in the magnetization of the rocks in the Earth's crust). Using flight trajectories recorded by GPS-based tracking devices, they found that many of the pigeons released at unfamiliar sites initially flew, sometimes several kilometers, in directions that were either parallel or perpendicular to the bearing of the local intensity field (Figure 22). Pigeons exhibited this behavior regardless of the bearing of their home loft and significantly more often than would be expected by chance. These results provide evidence that pigeons are able to detect spatial variation in the strength of the Earth’s magnetic field. By aligning their flight paths either parallel or perpendicular to lines of magnetic field strength, pigeons might be ‘sampling’ the strength and variability of the local magnetic field, information that could potentially be useful for navigation (Dennis et al. 2007).
Wiltschko et al. (2009) also released homing pigeons near a magnetic anomaly and found that the irregular magnetic field in the area of the anomaly caused confusion. Pigeons took longer than normal to decide which direction to finally orient (termed the vanishing interval in homing pigeon research). Because other orientation cues are not affected by magnetic anomalies (i.e., the inclination compass and the sun compass), such results indicate that the pigeons detected and apparently confused by the anomalous magnetic intensity. This, in turn, indicates that homing pigeons normally ‘record’ and use information about magnetic intensity and its changes as part of their navigational process. However, in contrast to Dennis et al. (2007), Wiltschko et al. (2009) found no evidence that the pigeons in their study followed gradients of magnetic intensity. Thus, although there is evidence that at least some birds can ‘sense’ magnetic intensity, additional studies of pigeons and other species of birds are needed to determine if and to what extent birds might use gradients of magnetic intensity for navigation.

Figure 22. Examples of the orientation of flight trajectories of homing pigeons relative to isopleths of geomagnetic intensity.
Single-color lines and points indicate flight trajectories and position fixes of individual pigeons. Yellow circles show the location of
release sites, and yellow lines designate the straight-line direction to the home loft. Thin green lines are magnetic intensity isopleths
(10 nT intervals). Background color depicts relative elevation (low-elevation areas are blue and high-elevation areas are green). Red
scale bars are 500 m. Arrows indicate locations of alignment of individual birds: ( a) Long-distance alignment along lines of similar magnetic
intensity; ( b, c) Parallel and/or perpendicular alignments at two other release sites; ( d– f). Detailed views of examples of parallel and
perpendicular alignments (From: Dennis et al. 2007).
A study where White-crowned Sparrows were displaced longitudinally across distances ranging from 266 to 2869 kilometers provides some additional possible clues about how magnetic cues might be used by birds (Åkesson et al. 2005). Both young and adult White-crowned Sparrows were captured on their breeding area in the Northwest Territories, Canada, towards the end of the breeding season and shortly before they would normally begin fall migration (15 July to 10 August). One group of sparrows (15 adults and 15 juveniles) were transported (on an icebreaker) to unfamiliar sites along a northeasterly route to the magnetic north pole (79.0° N, 105.1° W) and then further southeast (Figure 23). A control group (5 adults and 39 juveniles) was transported a short distance west of the capture site. Using Emlen funnels (Figure 6 above), the directional orientation of both groups of sparrows was determined, with the experimental group tested at nine different locations (including their breeding area; site 1 in the Figure 23B). Sparrows in the control group generally oriented to the southeast (Figure 24), the general direction of their presumed wintering area.
Sparrows being transported to the east, however, shifted their orientation from their normal migratory direction to a direction leading back to the breeding area or their typical migration route, suggesting compensation for the west-to-east displacement using geomagnetic cues (perhaps in combination with solar cues). One possible cue used by the sparrows is geomagnetic declination (the angle formed by the difference between geographic and magnetic north). The White-crowned Sparrows could have used the stars to determine geographic north (i.e., rotation center of sky) and their inclination compass to determine magnetic north. Determining the exact angle of declination at high latitudes is likely difficult because of the steep geomagnetic field lines, but recognizing a positive versus a negative declination would be easier. If so, the area near the point of zero declination could have been used as a longitudinal cue that, when passed, could have triggered a shift in orientation. Because this study is the only one to date to even suggest the possibility that birds might use magnetic declination as a navigational cue, additional studies of other species are needed to further examine the possible importance of declination as a navigational cue for birds.
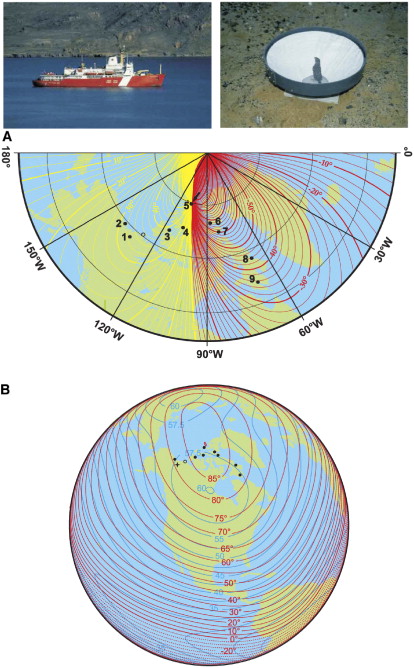
Figure 23. Sites where orientation cage experiments with White-crowned Sparrows were conducted in 1999 by
Åkesson et al. (2005). Sparrows were captured in the breeding area (site 1), and their orientation was recorded in circular
cages at sites 1–9. At one site (open circle), the birds were transported to the tundra, but no experiments were performed because
of high speeds. In 1999, the geomagnetic North Pole was located at Ellef Ringnes Island (site 5, arrow in [A]). (A) The map
showing magnetic declination. Yellow isolines indicate positive declination (deviations to the east of geographic north) and red
negative (deviations to the west) values, respectively. (B) Map showing isoclinics (geomagnetic inclination) as red (broken) lines
and isodynamics (total field intensity, µT) as blue (filled) lines. The star indicates the site of capture (From: Åkesson et al. 2005)

Figure 24. Orientation of displaced White-crowned Sparrows. A-G, adults; H-N, juveniles. Control birds and those tested at
a location west of the capture location on the breeding grounds typically oriented to the east or southeast in the general direction
of their wintering area. Sparrows transported in an icebreaker east of the capture site tended to oriented to the west or northwest, a
direction that would lead them back to the breeding area or their typical migration route (From: Åkesson et al. 2005).
Although empirical evidence is lacking, as an example of how other magnetic cues could be used for navigation by birds, the lines of equal intensity and equal vertical field intensity form a grid-like pattern in most areas, with the two gradients typically differing by about 10 to 30 degrees (Gould 1998; Figure 25). In addition, other ‘isomagnetics’ also intersect, to varying degrees depending on location, to form gradients (Figure 26). Although the magnetite-based receptors of birds appear to be sufficiently sensitive to potentially use such gradients for navigational purposes, the possibility that birds might use such cues for navigational purposes remains to be determined.
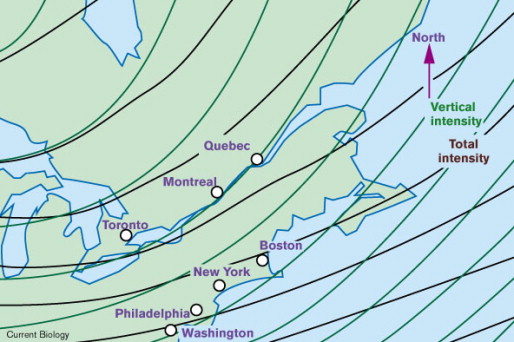
Figure 25. The strengths of both total field intensity and vertical field intensity increase toward the poles, but the axes of the
gradients typically diverge by 10–30°, as shown here for northeastern North America. In theory, any bicoordinate grid can be
used to specify location, though the resolution is best when the axes diverge by 90°. To use magnetic gradients, an animal would
have to measure both the rate of change and direction of change of each gradient in a local area. The larger the area of
experience, the more likely that these measurements will be representative of the world in general. (From: Gould 1998).
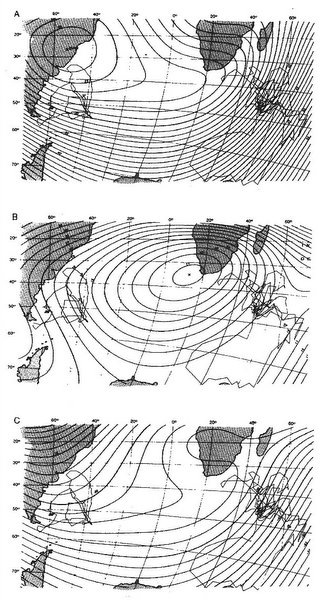 |
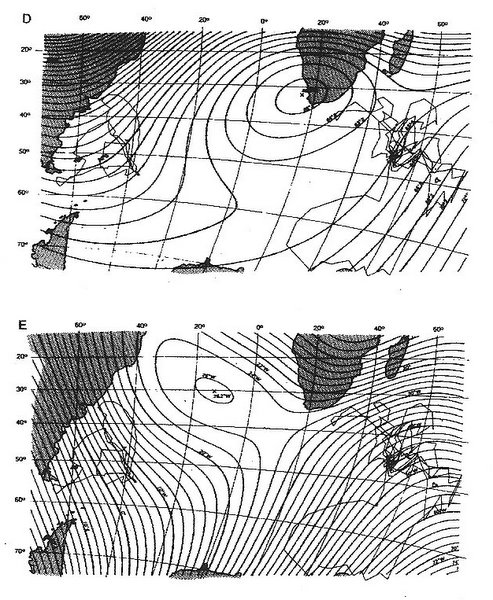 Figure 26. The isomagnetics for (A) total field intensity, (B) horizontal field intensity, (C) vertical field intensity, (D) inclination, and (E) declination in a portion of the southern hemisphere including parts of South America and southern Africa. Birds navigating in the area could potentially use two or more of these cues (particularly those that intersect at an angle to form grid-like patterns) to generate a magnetic navigational map (From: Åkesson and Alerstam 1998). |
Long- and short-range navigation
Available evidence clearly indicates that many, if not most, birds have a variety of ‘compasses’ that can be used for orientation and, although how they do it remains a question, are also able to determine their location. Thus, many birds can determine where they are and then select a course or direction that will take them to their goal. When selecting that direction, birds can potentially choose from among a variety of compass mechanisms, including celestial cues (e.g., star compass, sun compass, and polarized light) and magnetic cues (e.g., inclination compass). When moving closer to a goal, birds can also potentially use landmarks, olfactory cues, or even infrasounds. Given this array of potentially available cues, how do birds use or integrate the information from the various cues to find their way?
The availability of different compass mechanisms changes with time of day (e.g., sun and star compasses) and weather conditions. When multiple cues are potentially available, birds may preferentially use one compass or may integrate the information from, for example, the inclination compass and the celestial compass. Birds may also use one compass cue to calibrate another cue. Such calibration may be critical for maintaining an accurate heading because cue availability changes with weather conditions, season, time of day, and latitude, and directional information between different compass systems sometimes diverge (e.g., because magnetic declination, the difference between magnetic and geographic north, varies with location). As a result, birds must calibrate the different compasses with respect to a common reference both before and during migration to avoid navigational errors.
As an example of preferential use of compass cues, clock-shift experiments indicate that homing pigeons use the sun as their preferred compass, using magnetic cues when the sun is not available (e.g., cloudy days; Walcott 2005). Experiments with night-migrating Gray-cheeked (Catharus minimus) and Swainson’s (C. ustulatus) thrushes revealed that they depended primarily on sunset cues (the location of sunset or polarized light), using the solar twilight azimuth to calibrate their magnetic compass (Cochran et al. 2004). Temporarily-caged thrushes exposed to an east-pointing magnetic field, but allowed to see the setting sun, apparently ignored the magnetic field and still oriented north. However, based on this experience, they learned that the direction ‘north’ (as indicated by the setting sun) was rotated 90 degrees counterclockwise from the magnetic field. When subsequently released after sunset (and, out of their cages, now experiencing the normal magnetic field) with radio-transmitters so they could be followed, they flew west, mistakenly still assuming that they needed to orient 90 degrees counterclockwise from the magnetic field to head north (Fig. 27). Fortunately, the next evening, the radio-tagged thrushes used both cues (the normal magnetic field and observing sunset), determined which direction was north, and flew in the northerly direction that would take them to their breeding areas (Figure 28). Interestingly, some experimental Gray-cheeked Thrushes, when released and perhaps uncertain about their proper heading because the magnetic and sunset cues conflicted, did not immediately resume migration (Figure 28).
 ]
]
Figure 27. If the solar twilight azimuth is used to calibrate the magnetic compass on a daily basis, then (A) thrushes experiencing a normal magnetic field and observing sunset should orient north, (B) thrushes experiencing a magnetic field turned toward the east and observing sunset should determine that they need to orient 90 degrees counterclockwise to head north, (C) thrushes who have experienced a magnetic field turned toward the east and observed sunset and determine that they need to orient 90 degrees counterclockwise to head north should, if experiencing the normal magnetic field without an opportunity to view sunset, orient toward the west because they’ve ‘learned’ to orient 90 degrees counterclockwise relative to the magnetic field, and (D) on subsequent nights when exposed to both the normal magnetic field and sunset, they should return to their normal northerly migratory direction (From: Cochran et al. 2004).
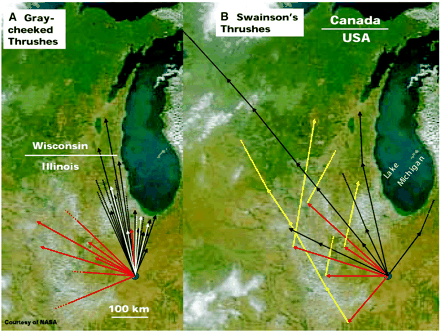
Figure 28. Tracks of free-flying (A) Gray-cheeked Thrushes and (B) Swainson's Thrushes. Arrows indicate the direction and ground track of flights. Data are depicted differently in (A) and (B) because for Gray-cheeked Thrushes experimental and control birds are different individuals, whereas in Swainson's Thrushes the same experimental individuals were followed for at least two successive nocturnal migrations (because of the large spread in natural headings). Connected arrows show flights of the same individual during successive nights. Natural migratory flights are indicated by the black arrows; flights where thrushes were exposed to magnetic fields turned east before takeoff (and not allowed to see sunset) are indicated by red arrows. For Swainson’s Thrushes, flights of experimental birds on the second night after release are indicated by yellow arrows. For Gray-cheeked Thrushes, birds exposed to the ‘east’ magnetic field that were released, did not migrate on the first night after release, but did so 1 to 6 days later are indicated by white arrows. Broken lines indicate that birds were lost during tracking at the site where the broken lines start (From: Cochran et al. 2004).
Migrating Swainson’s and Gray-cheeked thrushes used one cue (sunset) to calibrate a second cue (magnetic north) and similar behavior has been reported by other birds. For example, migrating Savannah Sparrows (Passerculus sandwichensis) use polarized light cues to calibrate their magnetic (inclination) compass (Muheim et al. 2006, 2007). Other studies have revealed that some species of birds appear to use their magnetic compass to calibrate star and sunset cues (Sandberg et al. 2000). More generally, Sandberg et al. (2000) suggested that migrating birds may depend primarily on their magnetic (inclination) compass when faced with long-distance, non-stop flights across inhospitable terrain, such as deserts or large bodies of water. Migrating birds making shorter flights over more hospitable areas may preferentially use celestial cues calibrated using the magnetic field. Birds might exhibit such differences because, in contrast to celestial cues, the inclination compass is always available, regardless of time of day or weather conditions. Clearly, when traveling over large, inhospitable areas, a bird losing access to its primary compass because of changing weather conditions (e.g., increased cloud cover) and becoming disoriented may not survive.
In addition to preferentially using certain compass cues to calibrate others, for long-range navigation, migrating birds may use different compass cues at different times or different locations. For example, Bingham and Cheng (2005) suggested that, for long-distance navigation, birds might first determine their location relative to their ‘goal’ on a mental,’ bi-coordinate map that could potentially be generated, as described previously, using magnetic cues (Figure 29). Initial orientation toward that goal could involve multiple compass mechanisms, including the use of celestial (stars and sun) and magnetic (inclination compass) cues. A bird getting closer to its goal and entering more familiar areas may rely more on regional or local cues such as odors (Figure 29), major landmarks (such as mountains, rivers, coastlines, or lakes), or even infrasound. When nearing the location of its goal, a bird would likely use finer landscape features such as general topography, patterns generated by areas or patches of different habitat types, bodies of water, and, in some areas, man-made features such as roads. Finally, birds likely use specific features of or landmarks in their ‘goal’ area, such as their breeding territory or wintering area, to determine that they have arrived at the desired destination.

Fig. 29. A global-navigation mechanism based on the use of multiple cues at different scalar resolutions (Bingman and Cheng 2005). As an example, an experienced migrant begins its spring migration in South America and heads in an approximate direction toward the breeding site in Ontario, Canada, exploiting low-resolution bi-coordinate, grid-based navigation relying on variations in the Earth’s magnetic field (Phase 1). As the migrant approaches its breeding area, control of its navigational behavior switches to a higher-resolution, bi-coordinate, grid-based navigation based on variation in atmospheric odors (Phase 2). Getting closer to its breeding site, control of the migrant’s navigational behavior again switches to even higher-resolution map-based navigation based on familiar visual landmarks (Phase 3). The migratory journey closes as the bird beacons in on its breeding territory of the previous year (Phase 4). The spatial resolution of the different navigational mechanisms can be interpreted using the inset to the lower left. The increasing accuracy of successive phases is indicated by narrower probability distributions around the target (goal) direction. Thus, the later phases are more likely to get the bird close to its goal than the earlier phases. (From: Bingman and Cheng 2005).
Able, K. P. 2000. The concepts and terminology of bird navigation. Journal of Avian Biology 32: 174-183.
Able, K. P., and M. A. Able. 1996. The flexible migratory orientation system of the Savannah Sparrow (Passerculus sandwichensis). Journal of Experimental Biology 199: 3-8.
Åkesson, S., and T. Alerstam. 1998. Oceanic navigation: are there any feasible geomagnetic bi-coordinate combinations for albatrosses? Journal of Avian Biology 29: 618-625.
Åkesson, S., J. Morin, R. Muheim, and U. Ottosson. 2005. Dramatic orientation shift of White-crowned Sparrows displaced across longitudes in the High Arctic. Current Biology 15: 1591-1597.
Beason, R. C. 2004. What can birds hear? Proceedings of the Vertebrate Pest Conference 21: 92-96.
Beason, R. C. 1987. Interaction of visual and non-visual cues during migratory orientation by the Bobolink (Dolichonyx oryzivorus). Journal für Ornithologie 128: 317-328.
Beason, R. C. 1992. You can get there from here: responses to simulated magnetic equator crossing by the Bobolink (Dolichonyx oryzivorus). Ethology 91:75-80.
Beason, R. C. 2005. Mechanisms of magnetic orientation in birds. Integrative and Comparative Biology 45: 565–573.
Bildstein, K. L. 2006. Migrating raptors of the world: their ecology and conservation. Cornell University Press, Ithaca, NY.
Bingman, V. P., K. P. Able, and P. Kerlinger. 1982. Wind drift, compensation, and the use of landmarks by nocturnal bird migrants. Animal Behaviour 30: 49-53.
Bingman, V. P., and K. Cheng. 2005. Mechanisms of animal global navigation: comparative perspectives and enduring challenges. Ethology Ecology & Evolution 17: 295-318.
Bruderer, B., and F. Liechti. 1999. Bird migration across the Mediterranean. In: Proceedings XXII International Ornithology Congress (N. J. Adams and R. H. Slotow, eds.), pp. 1983-1999. University of Natal, Durban, South Africa.
Chernetsov, N., D. Kishkinev, and H. Mouritsen. 2008. A long-distance avian migrant compensates for longitudinal displacement during spring migration. Current Biology 18: 188-190.
Cochran, W. W., H. Mouritsen, and M. Wikelski. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304:405-408.
Dennis, T. E., M. J. Rayner, and M. M. Walker. 2007. Evidence that pigeons orient to geomagnetic intensity during homing. Proceedings of the Royal Society B 274: 1153-1158.
Duff, S. J., L. A. Brownlie, D. F. Sherry, and M. Sangster. 1998. Sun compass and landmark orientation by Black-capped Chickadees (Parus atricapillus). Animal Behavior Processes 24: 243-253.
Emlen, S. T. 1966. A technique for recording migratory orientation of captive birds. Auk 83: 361-367.
Emlen, S. T. 1967a. Migratory orientation in the Indigo Bunting Passerina cyanea. Part I: evidence for use of celestial cues. Auk 84: 309-342.
Emlen, S. T. 1967b. Migratory orientation in the Indigo Bunting Passerina cyanea. Part II: Mechanism of celestial orientation. Auk 84: 463-489.
Emlen, S. T. 1969. The development of migratory orientation in young Indigo Buntings. Living Bird 1969: 716-718.
Emlen, S. T. 1970. Celestial rotation: its importance in the development of migratory orientation. Science 170: 1198-1201.
Freire, R., U. H. Munro, L. J. Rogers, R. Wiltschko, and W. Wiltschko. 2005. Chickens orient using a magnetic compass. Current Biology 15: R620-R621.
Gagliardo, A., P. Ioalè, M. Savini, H.-P. Lipp, and G. Dell'Omo. 2007. Finding home: the final step of the pigeons’ homing process studied with a GPS data logger. Journal of Experimental Biology 210: 1132-1138.
Gagliardo, A., G. Vallortigara, D. Nardi, and V. P. Bingman. 2005. A lateralized avian hippocampus: preferential role of the left hippocampal formation in Homing Pigeon sun compass-based spatial learning. European Journal of Neuroscience 22: 2549-2559.
Gould, J. L. 1998. Sensory bases of navigation. Current Biology 8: R731-R738.
Greenwood, V. J., E. L. Smith, S. C. Church, and J. C. Partridge. 2003. Behavioural investigation of polarization sensitivity in the Japanese Quail (Coturnix coturnix japonica) and the European Starling (Sturnus vulgaris). Journal of Experimental Biology 206: 3201-3210.
Hagstrum, J. T. 2000. Infrasound and the avian navigational map. Journal of Experimental Biology 203: 1103-1111.
Hein, C. M., M. Zapka, D. Heyers, S. Kutzschbauch, N.-L. Schneider, and H. Mouritsen. 2009. Night-migratory Garden Warblers can orient with their magnetic compass using the left, the right or both eyes. Journal of the Royal Society Interface, online early.
Heyers, D., M. Manns, H. Luksch, O. Güntürkün, and H. Mouritsen. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2(9): e937.
Holland, R. A., K. Thorup, A. Gagliardo, I. A. Bisson, E. Knecht, D. Mizrahi, and M. Wikelski. 2009. Testing the role of sensory systems in the migratory heading of a songbird. Journal of Experimental Biology 212: 4065-4071.
Huttunen, M. J. 2009. Magnetic and sunset orientation in migratory Redwings, Turdus iliacus. Italian Journal of Zoology 76: 133-142.
Jorge, P. E., P. A. M. Marques, and J. B. Phillips. 2010. Activational effects of odours on avian navigation. Proceedings of the Royal Society B, online early.
Kamil, A. C., and K. L. Gould. 2008. Memory in food caching animals. Papers in Behavior and Biological Sciences, University of Nebraska, Lincoln, NE.
Klaassen, M. 1996. Metabolic constraints on long-distance migration in birds. Journal of Experimental Biology 199: 57-64.
Kramer, G. 1950. Weitere analyse der faktoren, welche die zugaktivität des gekäfigten vogels orientieren. Naturwissenschaften 37: 377-378.
Kramer, G. 1953. Wird die Sonnenhöhe bei der Heimfindeorientierung verwertet? Journal für Ornithologie 94: 201-219.
Kramer, G. 1957. Experiments on bird orientation and their interpretation. Ibis 99: 196-227.
Kreithen, M. L., and D. B. Quine. 1979. Infrasound detection by the homing pigeon: a behavioral audiogram. Journal of Comparative Physiology 129: 1-4.
Liechti, F., D. Peter, R. Lardelli, and B. Bruderer. 1996. Herbstlicher Vogelzug im Alpenraum nach mondbeobactungen-topographie und wind beeinflussen den Zugverlauf. Der Ornithologis-che Beobachter 93:131-152.
Lohmann, K. J., C. M. F. Lohmann, and C. S. Endres. 2008. The sensory ecology of ocean navigation. Journal of Experimental Biology 211: 1719-1728.
Lohmann, K. J., C. M. F. Lohmann, and N. F. Putman. 2007. Magnetic maps in animals: nature’s GPS. Journal of Experimental Biology 210: 3697-3705.
Lohmann, K. J., N. F. Putman, and C. M. F. Lohmann. 2008. Geomagnetic imprinting: a unifying hypothesis of long-distance natal homing in salmon and sea turtles. Proceedings of the National Academy of Sciences USA 105: 19086-19101.
Miller, G. 2004. Behavioral neuroscience uncaged. Science 306:432-434.
Mora, C. V., M. Davison, J. M. Wild, and M. M. Walker. 2004. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature 432: 508-511.
Mouritsen, H. , G. Feenders, M. Liedvogel, K. Wada, and E. D. Jarvis. 2005. Night-vision brain area in migratory songbirds. Proceedings of the National Academy of Sciences USA 102: 8339-8344.
Mouritsen, H., G. F eenders, M. Liedvogel, and W. Kropp. 2004. Migratory birds use head scans to detect the direction of the earth's magnetic field . Current Biology 14: 1946 – 1949.
Mouritsen, H., U. Janssen-Bienhold, M. Liedvogel, G. Feenders, J. Stalleicken, P. Dirks, and R. Weiler. 2004. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proceedings of the National Academy of Sciences USA 101: 14294-14299.
Mouritsen, H., and T. Ritz. 2005. Magnetoreception and its use in bird navigation. Current Opinion in Neurobiology 15: 406-414.
Muheim, R., S. Åkesson, and J. B. Phillips. 2007. Magnetic compass of migratory Savannah Sparrows is calibrated by skylight polarization at sunrise and sunset. Journal of Ornithology 148: 485-494.
Muheim, R., J. B. Phillips, and S. Åkesson. 2006. Polarized light cues underlie compass calibration in migratory songbirds. Science 313: 837-839.
Muheim, R., J. B. Phillips, and M. E. Deutschlander. 2009. White-throated Sparrows calibrate their magnetic compass by polarized light cues during both autumn and spring migration. Journal of Experimental Biology 212: 3466-3472.
Nevitt, G. A. 2000. Olfactory foraging in Antarctic procellariiform seabirds: life at high Reynolds numbers. Biological Bulletin 198: 245-253.
Nevitt, G. A. 2008. Sensory ecology on the high seas: the odor world of the procellariiform seabirds. Journal of Experimental Biology 211: 1706-1713.
Nevitt, G. A., and F. Bonadonna. 2005. Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biology Letters 1: 303-305.
Perdeck, A. C. 1958. Two types of orientation in migrating Sturnus vulgaris and Fringilla coelebs as revealed by displacement experiments. Ardea 46: 1-37.
Piersma, T., L. Zwarts, and J. H. Bruggeman. 1990. Behavioural aspects of the departure of waders before long-distance flights: flocking, vocalizations, flight paths and diurnal timing. Ardea 78: 157-184.
Pomozi, I., G. Horváth, and R. Wehner. 2001. How the clear-sky angle of polarization pattern continues underneath clouds: full-sky measurements and implications for animal orientation. Journal of Experimental Biology 204: 2933-2942.
Pósafi, M., and R. E. Dunin-Borkowski. 2009. Magnetic nanocrystals in organisms. Elements 5: 235-240.
Ritz, T., S. Adem, and K. Schulten. 2000. A model for photoreceptor-based magnetoreception in birds. Biophysical Journal 78: 707-718.
Sandberg, R., J. B äckman, F. R. Moore, and M. L õhmus. 2000. Magnetic information calibrates celestial cues during migration. Animal Behaviour 60: 453-462.
Schmidt-Koenig, K. 1958. Der Einfluß experimentell veränderter Zeitschätzung auf das Heimfindevermögen bei Brieftauben. Naturwissenschaften 45: 47.
Semm, P., and R. C. Beason. 1990. Responses to small magnetic variations by the trigeminal system of the Bobolink. Brain Research Bulletin 25: 735-740.
Solov’yov, I. A., and W. Greiner. 2009a. Micromagnetic insight into a magnetoreceptor in birds: existence of magnetic field amplifiers in the beak. Physical Review E 80: 041919 (online).
Solov’yov, I. A., and W. Greiner. 2009b. Iron-mineral-based magnetoreceptor in birds: polarity or inclination compass? European Physical Journal D 51: 161-172.
Stapput, K., P. Thalau, R. Wiltschko, and W. Wiltschko. 2008. Orientation of birds in total darkness. Current Biology 18: 602-606.
Theurich, M., G. Langner, and H. Scheich. 1984. Infrasound responses in the midbrain of the Guinea Fowl. Neuroscience Letters 49:81-86.
Thorup, K. I.-A. Bisson, M. S. Bowlin, R. A. Holland, J. C. Wingfield, M. Ramenofsky, and M. Wikelski. 2007. Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proceedings of the National Academy of Sciences USA 104: 18115-18119.
Thorup, K., and R. A. Holland. 2009. The bird GPS – long-range navigation in migrants. Journal of Experimental Biology 212: 3597-3604.
Thorup, K., and J. Rabøl. 2007. Compensatory behaviour after displacement in migratory birds. Behavioral Ecology and Sociobiology 61: 825-841.
von Middendorff, A. 1859. Die isepiptesen Russlands. Mem. Acad. Sci. St. Petersbourg VI, Ser. Tome. 8:1–143.
Voss, J., N. Keary, and H.-J. Bischof. 2007. The use of the geomagnetic field for short distance orientation in Zebra Finches. NeuroReport 18: 1053-1057.
Walcott, C. 2005. Multi-modal orientation cues in homing pigeons. Integrative and Comparative Biology 45: 574-581.
Walker, M. M. 1998. On a wing and a vector: a model for magnetic navigation by homing pigeons. Journal of Theoretical Biology 192: 341-349.
Wallraff, H. G. 2000. Path integration by passively displaced homing pigeons? Animal Behaviour 60: F30-F36.
Wallraff, H. G. 2004. Avian olfactory navigation: its empirical foundation and conceptual state. Animal Behaviour 67: 189-204.
Wang, K., E. Mattern, and T. Ritz. 2006. On the use of magnets to disrupt the physiological compass of birds. Physical Biology 3:220-231.
Weindler, P., M. Baumetz, and W. Wiltschko. 1997. The direction of celestial rotation influences the development of stellar orientation in young Garden Warblers (Sylvia borin). Journal of Experimental Biology 200: 2107-2113.
Williams, T. C., J. M. Williams, P. G. Williams, and P. Stokstad. 2001. Bird migration through a mountain pass studied with high resolution radar, ceilometers, and census. Auk 118: 389-403.
Wiltschko, R., C. Haugh, M. Walker, and W. Wiltschko. 1998. Pigeon homing: sun compass use in the southern hemisphere. Behavioral Ecology and Sociobiology 43: 297-300.
Wiltschko, R., U. Munro, H. Ford, and W. Wiltschko. 2008. Contradictory results on the role of polarized light in compass calibration in migratory songbirds. Journal of Ornithology 149: 607-614.
Wiltschko, R., I. Schiffner, and W. Wiltschko. 2009. A strong magnetic anomaly affects pigeon navigation. Journal of Experimental Biology 212: 2983-2990.
Wiltschko, R., M. Walker, and W. Wiltschko. 2000. Sun-compass orientation in Homing Pigeons: compensation for different rates of change in azimuth? Journal of Experimental Biology 203: 889-894.
Wiltschko, R., and W. Wiltschko. 1981. The development of sun compass orientation in young homing pigeons. Behavioral Ecology and Sociobiology 9: 135-141.
Wiltschko, R. and W. Wiltschko. 2009. Avian navigation. Auk 126: 717-743.
Wiltschko, W. 1968. Über den Einfluβ statischer magnetfelder auf die zugorientierung der Rotkehlchen (Erithacus rubecula). Zeitschrift für Tierpsychologie 25:536-558.
Wiltschko, W., R Freire, U. Munro, T. Ritz, L. Rogers, P. Thalau, and R. Wiltschko. 2007. The magnetic compass of domestic chickens, Gallus gallus. Journal of Experimental Biology 210: 2300-2310.
Wiltschko, W., U. Munro, H. Ford, and R. Wiltschko. 2003. Lateralisation of magnetic compass orientation in Silvereyes, Zosterops lateralis. Australian Journal of Zoology 51: 1-6.
Wiltschko, W., U. Munro, H. Ford, and R. Wiltschko. 2006. Bird navigation: what type of information does the magnetite-based receptor provide? Proceedings of the Royal Society B 273: 2815-2820.
Wiltschko, W., J. Traudt, O. Güntürkün, H. Prior, and R. W iltschko. 2002. Lateralization of magnetic compass orientation in a migratory bird. Nature 419: 467-470.
Wiltschko, W., and R. Wiltschko.1998. The navigation system of birds and its development. In: Animal cognition in nature (R. P. Balda, I. M. Pepperberg, and A. C. Kamil, eds.), pp. 155–199. Academic Press, San Diego, CA.
Wolff, W. J. 1970. Goal orientation versus one-direction orientation in teal Anas c. crecca during autumn migration. Ardea 58: 131-141.
Wu, L.-Q., and J. D. Dickman. 2012. Neural correlates of a magnetic sense. Science, online early.
Zapka, M., D. Heyers, C. M. Hein, S. Engels, N.-L. Schneider, J. Hans, S. Weiler, D. Dreyer, D. Kishkinev, J. M. Wild, and H. Mouritsen. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461:1274-1277.