Secret routes of migratory birds
Bird Cast-Cornell Lab What's New in Bird Migration with Andrew Farnsworth
Molt migration
Molt is so demanding that some species migrate to different habitats to undergo the process- a phenomenon referred to as "molt migration." Investigators at the Institute of Bird Populations (IBP) in California have provided evidence that molt migration is more common than previously thought. Many songbirds were previously thought to molt on their breeding grounds prior to migration. And for many years, the phenomenon of molt migration was thought to occur in just a handful of species.
However, the IBP investigators reported evidence that many North American landbirds at least sometimes migrate or disperse to areas separate from the breeding or wintering grounds to molt. Examples include American Goldfinches, House Wrens, Carolina Wrens, Pacific Wrens, Gray Catbirds, and Northern Cardinals.
Why move from breeding areas to molt before migrating? A likely explanation is food availability. Birds breed in areas where food is abundant early in the summer, but, towards the end of the summer, availability of food resources (like insects) may decrease in those breeding areas. In other locations, however, food availability may increase and birds can move to such areas because more food means more energy to support the growth of new feathers (Source: Institute of Bird Populations).
Molt migration - waterfowl
More information about waterfowl molt migration - https://www.youtube.com/shorts/jqvHF7Atemw
Molt migration - songbirds
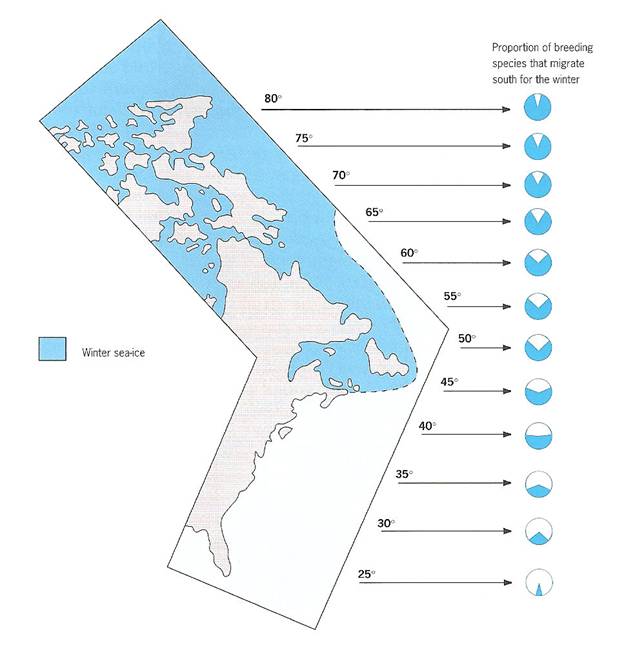
Figure 1. Proportion of breeding species at different latitudes in eastern North America that migrate south for the winter. Proportions range from about 12% for species that breed at 25°N to 87% for species breeding at 80°N, with the percentage increasing at a rate of 1.4% per degree of latitude (From: Newton 2003a, b).
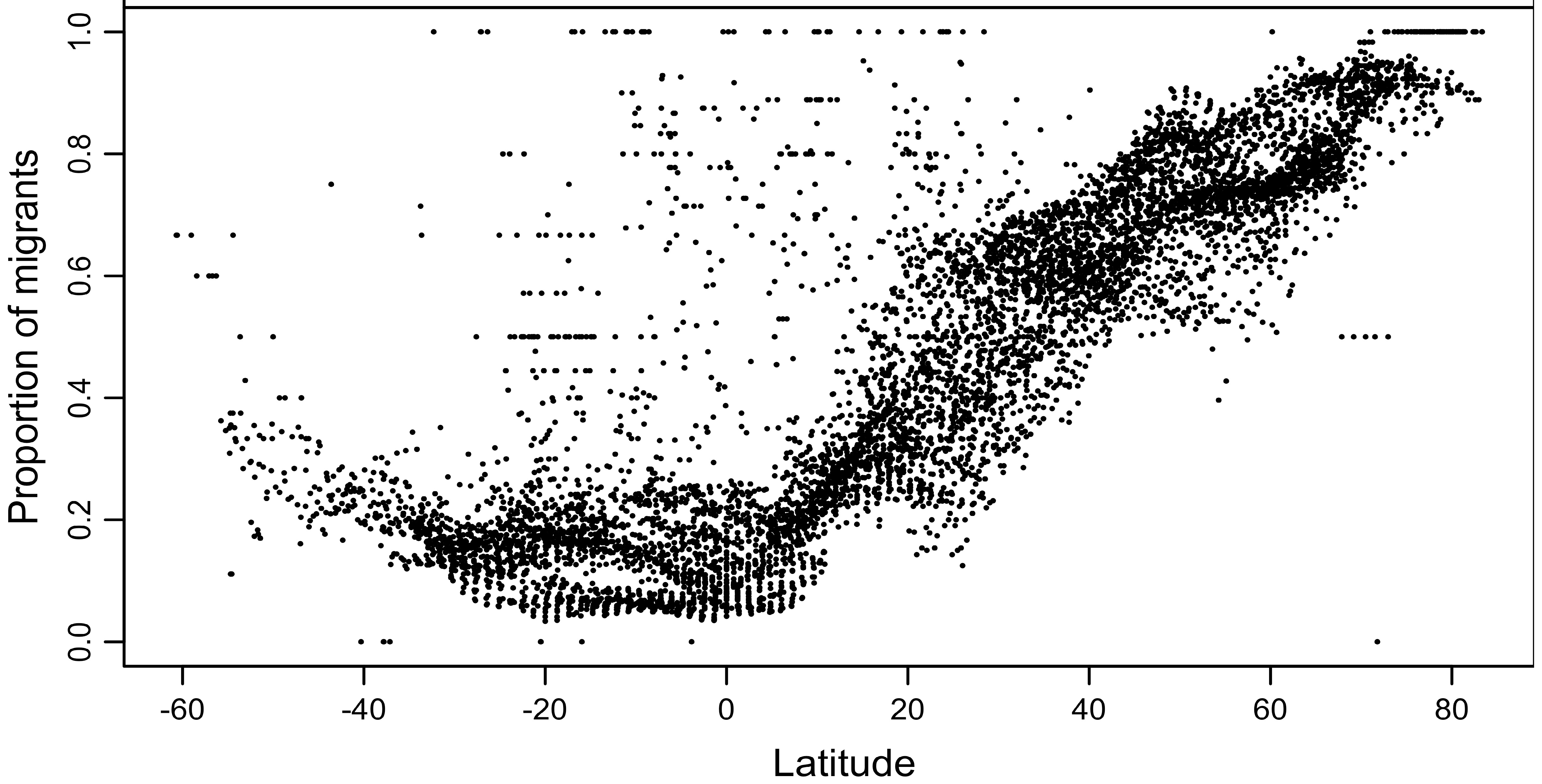
Latitudinal variation in the proportion of bird species that are migratory.
The proportion of migratory species is generally low south of the equator (generally <25%,
with a slight increasing trend towards the south), whereas in northern latitudes there is a constant and
marked increase from the equator northwards, reaching 100% around 80°N. (From: Somveille et al. 2013)
Before proceeding further, some definition of terms is needed. Migration involves movement of course, but not all bird movements can be called migration. Migration is the regular, endogenously controlled, seasonal movement of birds between breeding and non-breeding areas (Salewski and Bruderer 2007). Some birds make less predictable non-migratory movements in response to proximate environmental factors, but such movements are better termed irruptions or nomadic movements rather than migration. For example, whenever spruce seeds are scarce or absent over much of their normal breeding range in the boreal forests of the western Palearctic, large numbers of Common Crossbills (Loxia curvirostra) move long distances (often more than 1000 km) toward southern and western Europe (Newton 1972, Marquiss 2002). Such irruptions are irregular, but not infrequent; at least 40 such irruptions were known to have occurred during the 120-year period from 1881 to 2000 (Newton 2006). Other species of birds classified as irruptive species and whose movements are also triggered by declining food supplies include several that feed on seeds or fruit, such as Bramblings (Fringilla montifringilla) and waxwings (Bombycilla spp.), and some raptors, such as Snowy (Bubo scandiacus) and Great Gray (Strix nebulosa) owls.
Snowy Owl
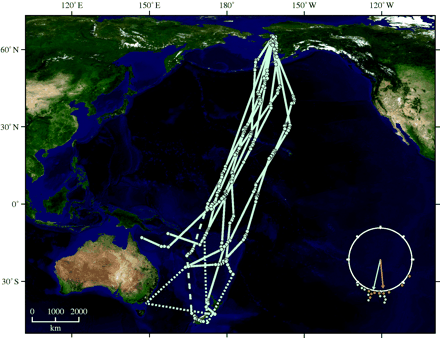
Many migratory birds are well known for their long-distance journeys. For example, many species of birds, such as Purple Martins, Common Nighthawks, Bobolinks, breed in North America and spend the winter in South America, with round-trip journeys of over 20,000 km. However, some migratory species of birds exhibit variation among populations or among individuals within populations in tendency to migrate and migration distances. For example, some species are partial migrants, with some individuals in a population migrating and others being sedentary. Other species exhibit differential migration, with differences among individuals in migration patterns based on age or sex. Still other species exhibit leap-frog migration, with populations or subspecies breeding at higher latitudes wintering further south (i.e., ‘leap-frogging’) than those breeding at lower latitudes (Figures 2 and 3). Finally, some species take different routes during spring and fall migration, a phenomenon known as loop migration (Figure 4). Thus, birds exhibit a diversity of migratory behaviors and many investigators have addressed the question of how such behaviors evolved.

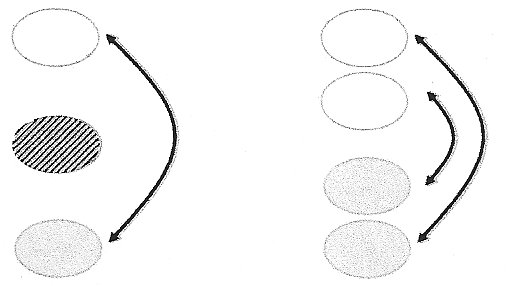
Figure 2. Examples of leap-frog migration. Left, individuals in a more northerly population migration to a location south of a
resident population of the same species. Right, all individuals in two breeding populations migrate, but those in the more northern
population migrate further and ‘leap-frog’ individuals from the more southerly breeding population (From: Boulet and Norris 2006).

Figure 3. Leap-frog migration by populations of Fox Sparrows. Several subspecies breed in southern Alaska
(una = Passerella iliaca unalaschensis, ins = P. i. insularis, and sin = P. i. sinuosa), others breed along with west coast of
Canada and the northwestern United States (ann = P. i. annectens, tow = P. i. townsendi, and ful = P. i. fuliginosa). The northernmost
subspecies have the southernmost wintering areas. P. i. fuliginosa is a resident, non-migratory subspecies. Solid lines indicate possible
trans-oceanic migration routes between breeding locations and the main wintering area in southern California (From: Bell 1997).
Winter connectivity and leapfrog migration by Painted Buntings -- Technological advances in migratory tracking tools have revealed a remarkable diversity in migratory patterns. One such pattern is leapfrog migration, where individuals that breed further north migrate to locations further south. Rueda-Hernández et al. (2023) analyzed the migration patterns of Painted Buntings (Passerina ciris) using a genetic-based approach. Their analyses of genetic variation across the breeding range revealed the existence of four genetically distinct groups within the species: Eastern, Southwestern, Louisiana, and Central groups. Subsequent assignment of migrating and wintering birds to genetic groups illustrated that birds from the Central group migrated during the fall via western Mexico or southern Texas, spent the winter from northeastern Mexico to Panama, and migrated north via the Gulf Coast of Mexico. Although Louisiana birds overlapped with Central birds on their spring migratory routes along the Gulf Coast, Louisiana birds had a more restricted wintering distribution in the Yucatan Peninsula and Central America. Further estimation of the straight-line distance from the predicted breeding location to the wintering location revealed that individuals sampled at lower winter latitudes traveled to greater distances (i.e., the predicted breeding area was further north), confirming that Painted Buntings exhibit a leapfrog migration pattern.
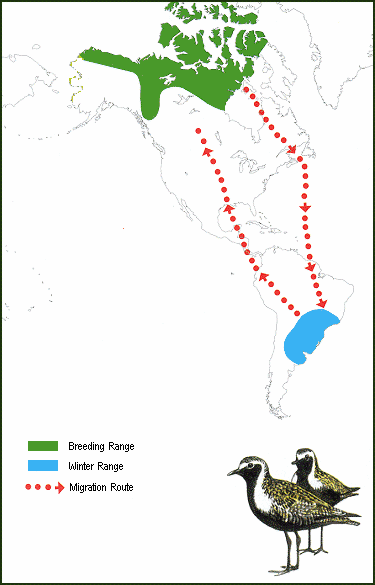
Figure 4. Migration routes of American Golden-Plovers. Many of these plovers take a non-stop
route across the Atlantic Ocean during fall migration, whereas they migrate further west during
spring migration; moving north through South and Central America, across the Gulf of Mexico
and through the United States and Canada to their breeding areas
(Source: http://www.npwrc.usgs.gov/resource/birds/migratio/patterns.htm).
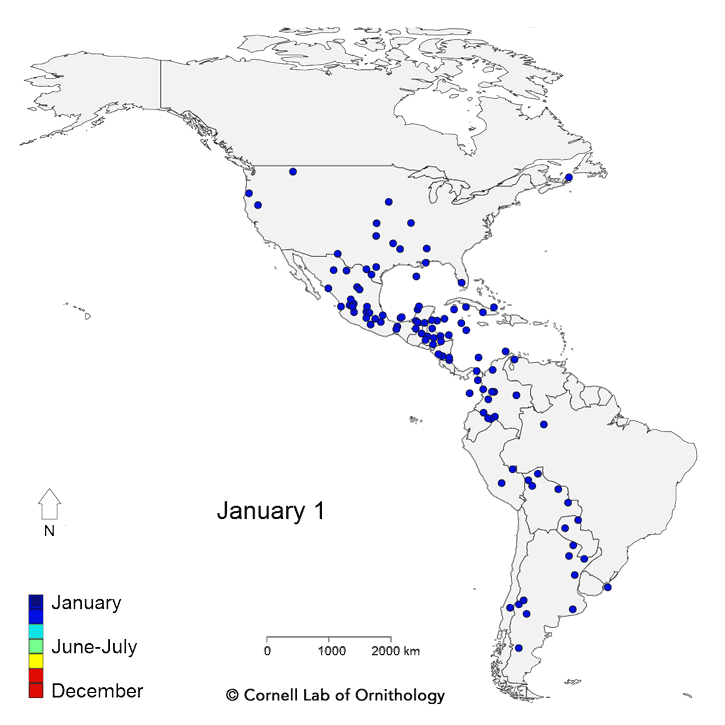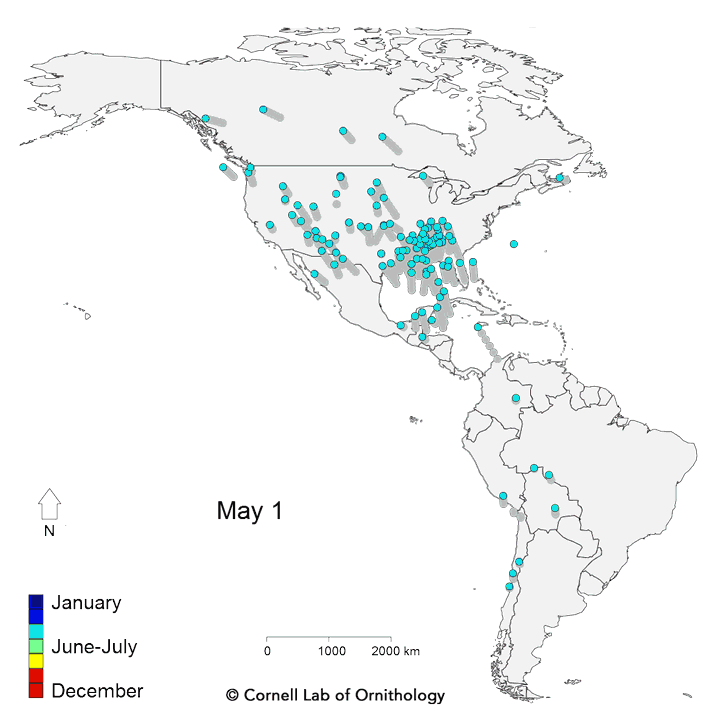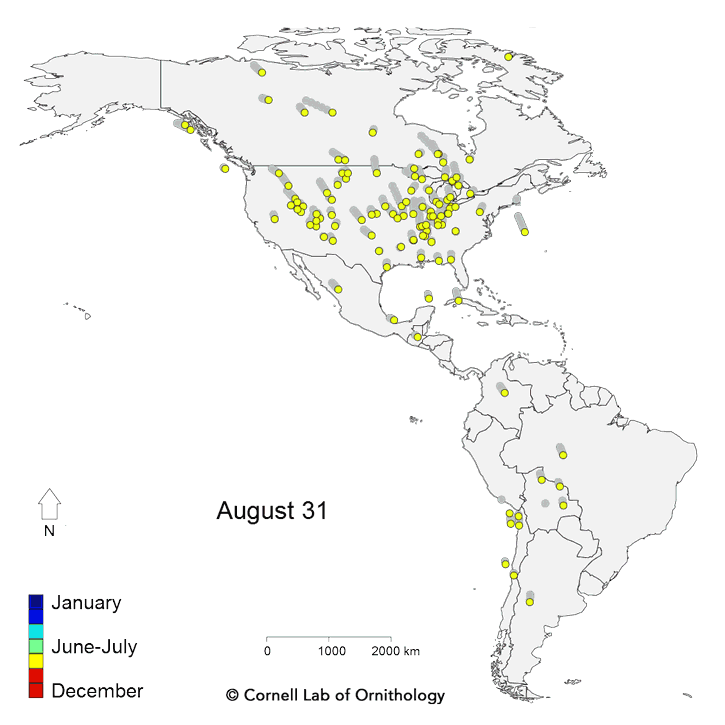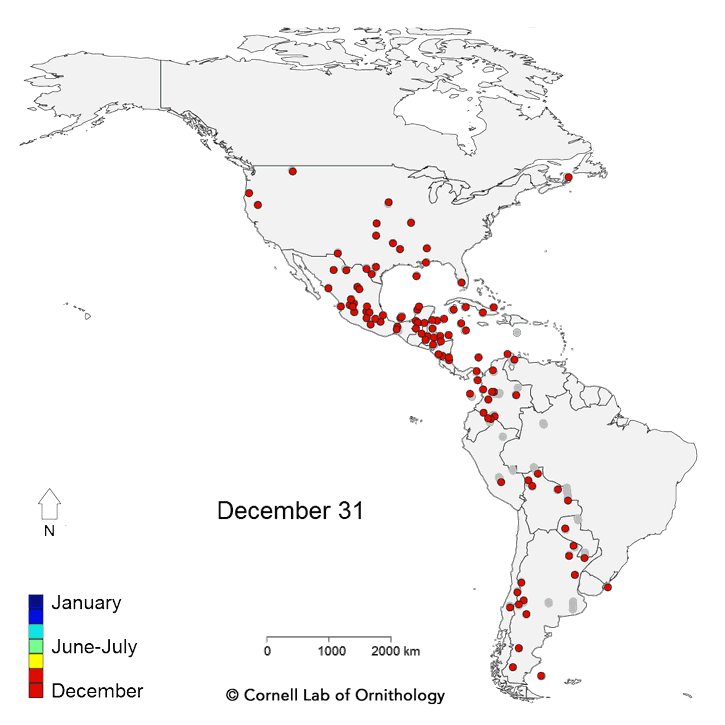
Migratory movements of 118 species of birds. Each dot represents a different species, with locations representing the average location of the population each day of the year.
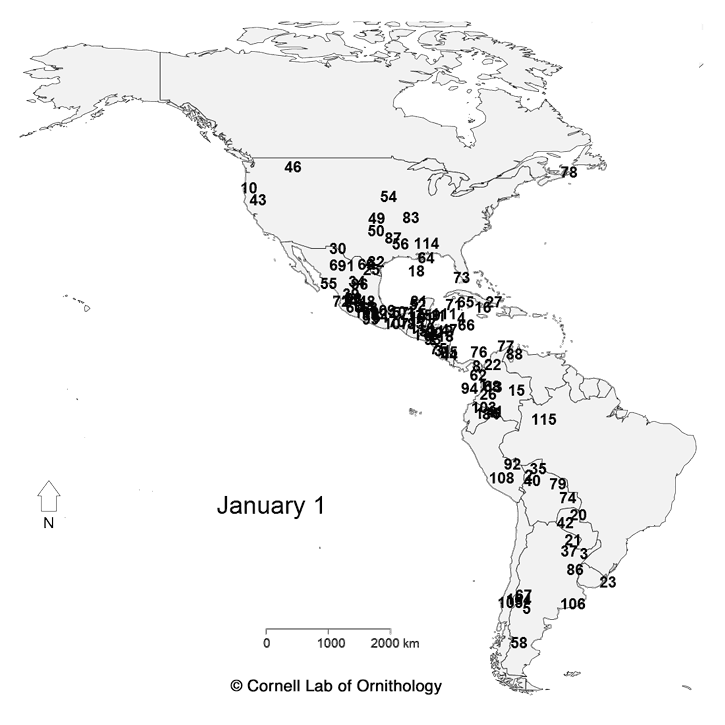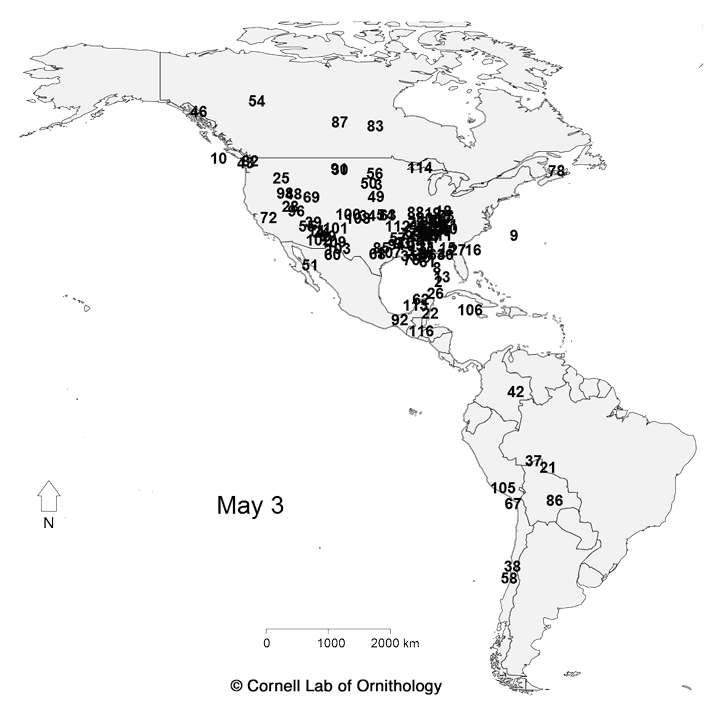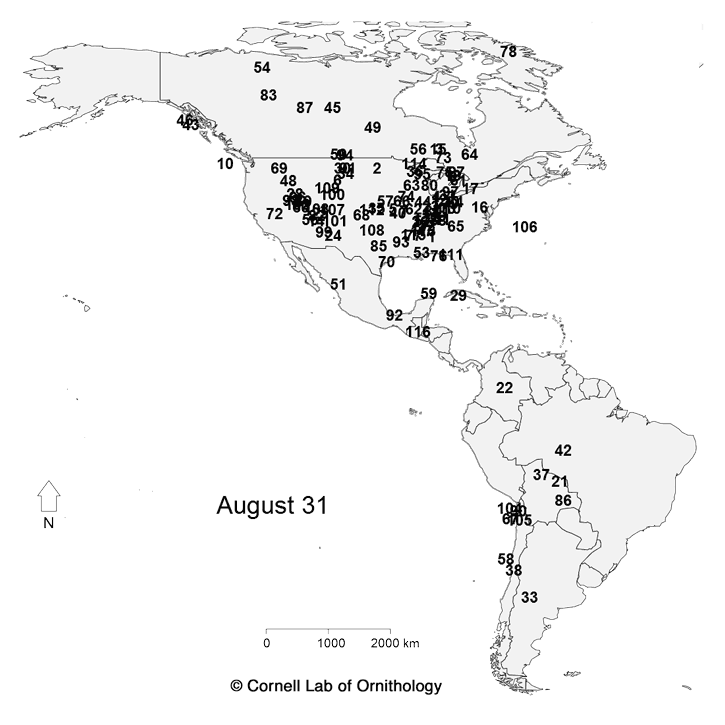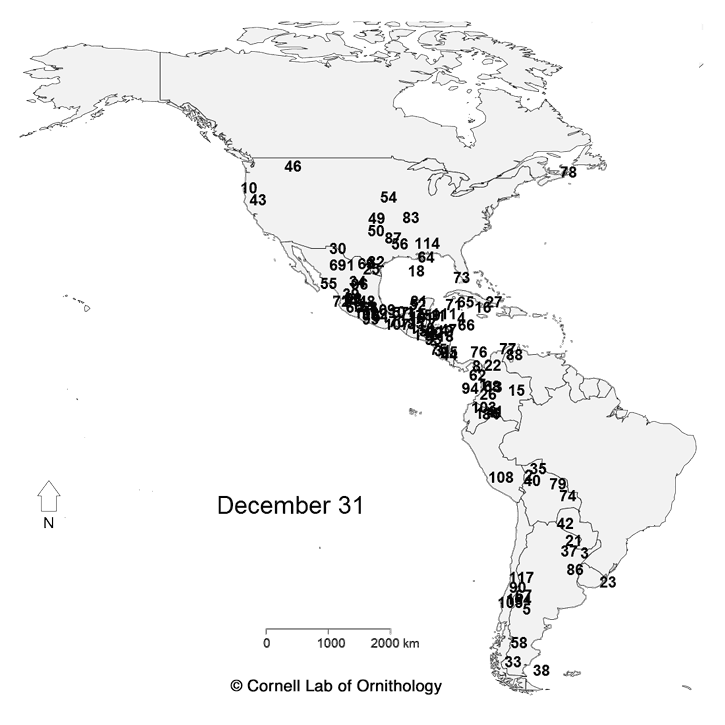 The key to which which species is which. |
|
60. MacGillivray’s Warbler |
For more information about the map, check
https://www.allaboutbirds.org/mesmerizing-migration-watch-118-bird-species-migrate-across-a-map-of-the-western-hemisphere/ and
http://rspb.royalsocietypublishing.org/content/283/1823/20152588
New Approaches for Understanding and Conserving Bird Migration --- Radar Ornithology in the Era of Big Data
Andrew Farnsworth
A study led by Smithsonian Migratory Bird Center and University of Alberta biologists has created a comprehensive picture of the 10,000 kilometer migratory route of
Common Nighthawks using GPS data. This study (Knight et al. 2021. Ecography 44: 665-679) is an important step in analyzing where and why nighthawk population numbers are declining.
Chain migration in swifts.
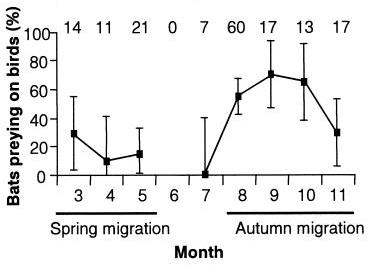
Although some migratory birds use a single wintering site, others make post-migratory movements during the nonbreeding season. Technological advances that enable tracking individual birds are uncovering more examples of post-migratory nonbreeding movements. Teitelbaumwe et al. (2023) reviewed the literature and found information about the post-migratory nonbreeding movements of 92 migratory bird species representing 18 orders across six continents. The most commonly reported reasons for these movements were resource availability and climate. Movement distances ranged from just a few km to over 1000 km. Birds were observed using up to 21 wintering sites in a single season, but the most common number of wintering sites was two and, in many studies, only part of the population made post-migratory nonbreeding movements. Resource-driven movements were most common for invertebrate-eaters and Passeriformes (songbirds), whereas climate-driven movements were more commonly reported for Anseriformes (waterfowl), Charadriiformes (shorebirds), and Phoenicopteriformes (flamingoes). Differentiating between climate as a proximate versus an ultimate driver of movement because of its effects on resource availability is difficult, but Teitelbaumwe et al. (2023) hypothesized that differences among species in the reasons for movement could be related to wintering habitat and constraints on site choice. Larger birds, like Anseriformes, might be more likely than smaller insectivores to overwinter in temperate regions, meaning that temperatures at their wintering sites fluctuate more and force force them to move. Also, temperatures might be more important for water-associated species (like waterfowl) because water freezes when temperatures drop below freezing (e.g, Common Pochards in the figure below).
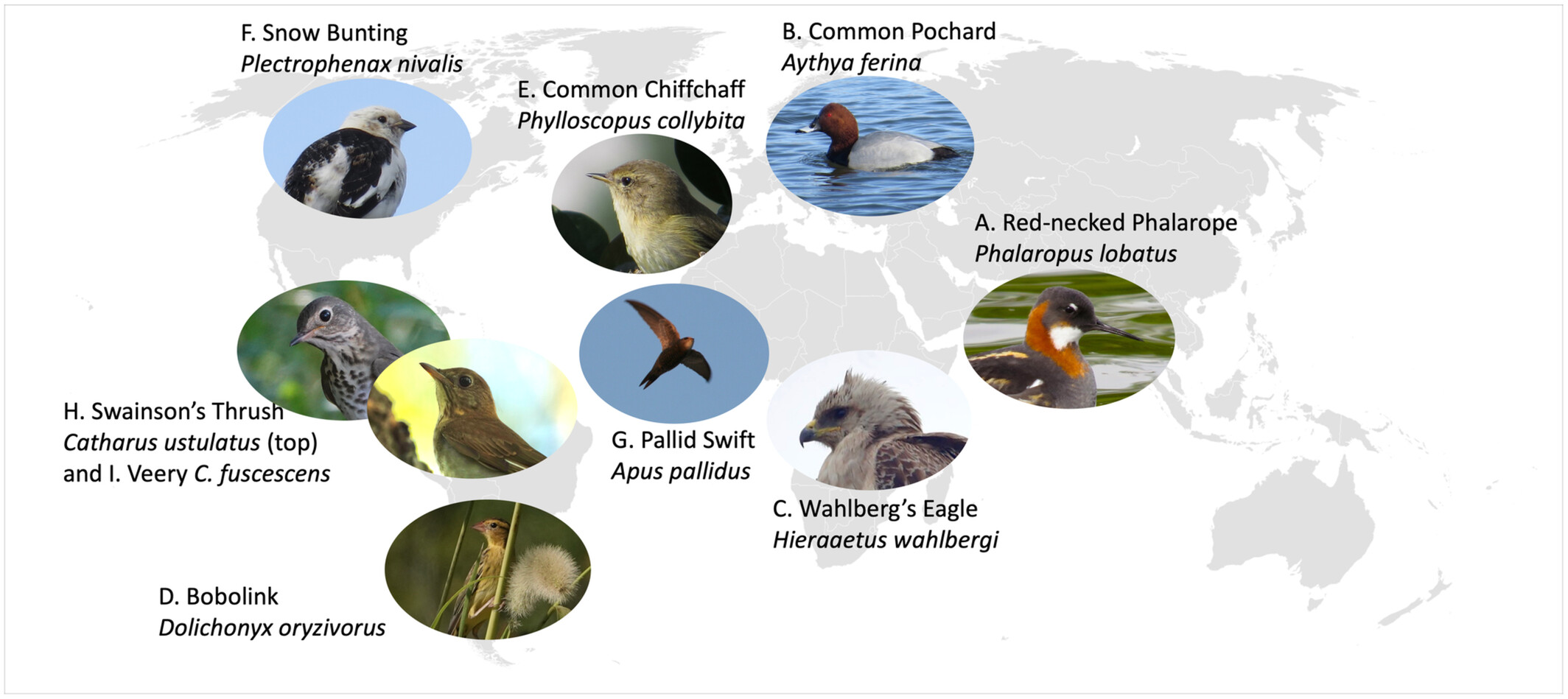
Examples of species that undertake post-migratory nonbreeding movements, illustrating
the diversity of taxa and geographic locations where such movments occur (From: Teitelbaumwe et al. 2023).
Mesoamerica (Mexico, Guatemala, Belize, El Salvador, Honduras, Nicaragua, and Costa Rica) - Important wintering sites for migratory birds
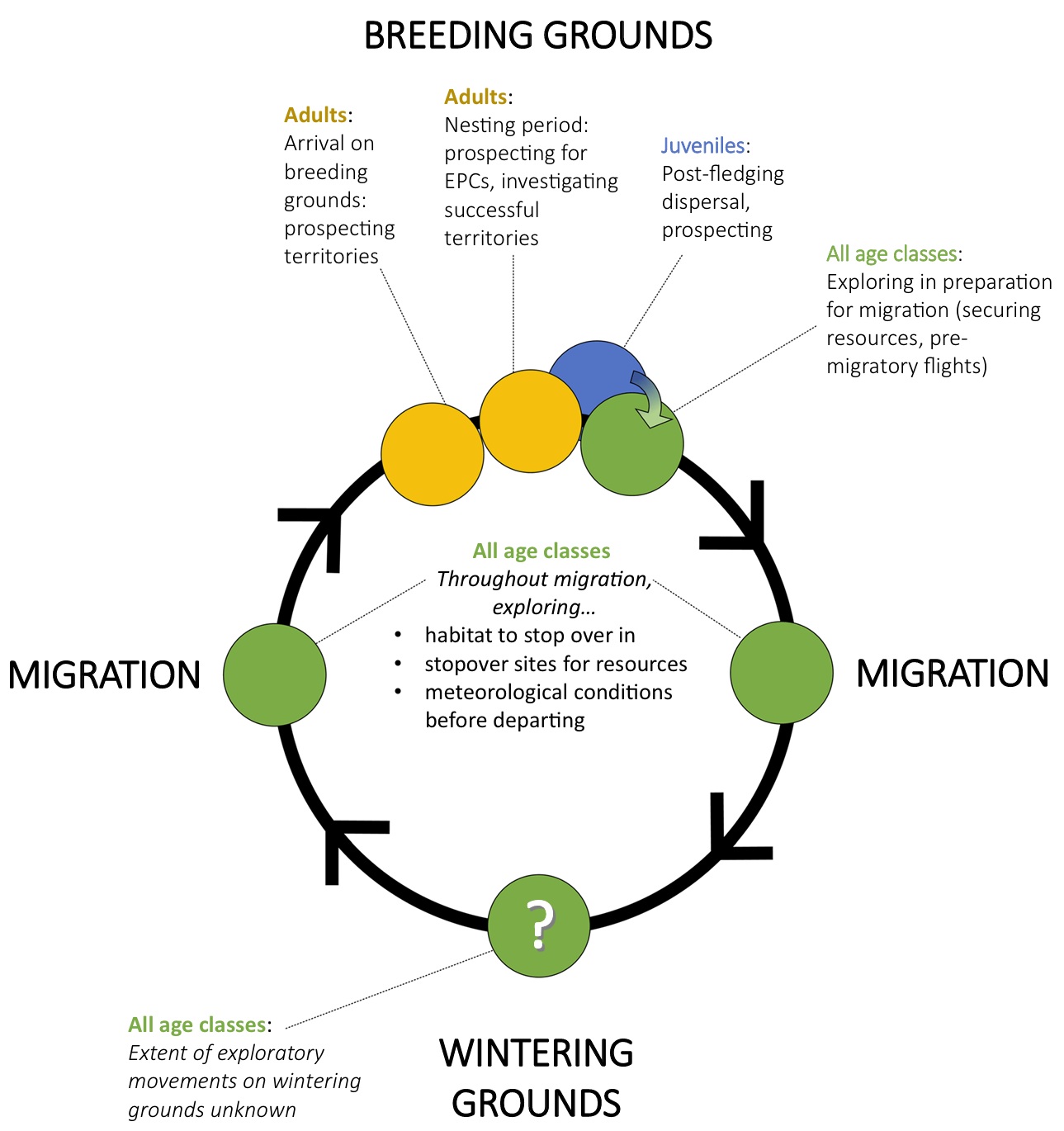
Exploratory movements have been observed in migratory songbirds across the annual cycle.
These movement types are found in all age classes. For example, on the breeding grounds,
a bird may prospect for extra-pair copulations or prospect other territories to evaluate how
successful they have been, while on migration, a bird may explore a stopover site to locate food.
Exploratory movements by birds can be defined as movements with a primary purpose to exploit nearby resources and/or gain information or experience beyond an individual’s current stationary phase, such as natal/ breeding area, wintering ground, or stopover site, to increase immediate (e.g., survival, extra-pair copulation) or delayed (e.g., information about favourable nest sites for future breeding season, etc.) fitness benefits. Such movements do not necessarily have to be directed with the primary purpose of relocating, but may happen in any direction and over different time periods.
During migration, most birds encounter novel environments. This is especially true for first year long-distance migrants, which often cross completely different ecosystems from their natal sites. Birds may need to adjust to unfamiliar conditions or seek the most favorable site within the novel environment, so exploratory movements can play an important role during the various stages of migration. After arriving at a stopover site, migrants may leave shortly afterwards in any direction to find a more suitable habitat within the “stopover landscape”. Individuals may make exploratory flights to gain familiarity within a patch, or may move continuously in an exploratory manner throughout a stopover. Northern Wheatears conduct exploratory flights on a stopover site prior to committing to departing, presumably to assess suitability of meteorological conditions aloft for the next migratory bout. These exploratory movements have direct consequences for birds’ fitness. Should a bird fail to find sufficient resources on stopover to fuel, it may starve. If it takes too long to accumulate energy, it may arrive at its breeding or wintering grounds too late to compete for a high-quality territory, reducing its indirect fitness. Should it depart in poor conditions, it may require more time to complete its migration, further reducing its probability of settling on a high-quality territory. As such, exploratory movements on stopover that serve to find favourable resources or assess meteorological conditions may have consequences both for an individuals’ immediate fitness (i.e. survival), but also its reproductive potential (From: Züst et al. 2023).
Extreme endurance flights during migration – Gill et al. (2009) used satellite telemetry to track the southward flights of Bar-tailed Godwits (Limosa lapponica baueri) from breeding areas in Alaska to wintering areas in New Zealand. Seven godwits with transmitters flew non-stop over distances ranging from 7008 to 11, 680 km across the Pacific Ocean. The duration of flights ranged from 5.0 to 9.4 days. These extraordinary non-stop flights establish new extremes for avian flight performance, roughly doubling the previous known maximum direct flight distance by birds. Maintaining an estimated metabolic rate of 8–10 times basal metabolic rate for more than 9 days represents a combination of metabolic intensity and duration that is unprecedented. Bar-tailed Godwits have several features that contribute to their ability to fly non-stop over such long periods, including efficient fuel consumption, an aspect ratio (9.2) that helps minimize lift-induced drag, and a well-streamlined body shape (Hedenström 2010).
For more information, check the video "Bar-tailed Godwits Nonstop Across the Pacific. |

Swainson's Thrush flying in a wind tunnel (Image credit: Science/AAAS)..
Proteins as a source of water during long-distance migration -- During migration, birds may travel thousands of kilometers between breeding and wintering grounds, stopping periodically to replenish fuel stores. The energy for flight is derived primarily from the oxidation of fatty acids stored in subcutaneous, abdominal, and intramuscular fat depots. Lean mass (mainly protein) is also catabolized, even while substantial fat stores remain, which results in reductions in the sizes of muscles and organs during flight. During migratory flights, high ventilation rates result in elevated rates of respiratory water loss and, as a result, dehydration, not fuel supply, may limit flight ranges under some conditions. However, catabolism of tissue protein yields five times as much water per kilojoule as fat, and so one proposed function of protein catabolism is to maintain water balance during nonstop flights. To test the protein-for-water hypothesis, Gerson and Guglielmo (2011) flew Swainson’s Thrushes (Catharus ustulatus) in a climatic wind tunnel under high- and low-humidity conditions at 18°C for up to 5 hours. Under moderately dry conditions, water loss in flight was sufficient to induce water production through increased protein catabolism. Moreover, the strong influence of the rate of water loss on the relative use of fat and protein indicates that use of fuel during flight is influenced by factors other than energy demand and that a physiological mechanism must exist where the extent of lean mass catabolism is influenced by ambient humidity and/or water stress. The protein-for-water strategy has clear functional significance for bird migration. The maintenance of water balance is an immediate necessity during migratory flight, and the use of protein to this end, within limits, allows birds to complete migratory flights in the face of unfavorable environmental conditions.
Migration routes of three Great Snipe. At left is their fall migration; at right,
spring migration (which is interrupted by a stopover in central Europe.
The long, fast migration of Great Snipes -- Migratory land birds perform extreme endurance flights when crossing ecological barriers, such as deserts, oceans and ice-caps. When travelling over benign areas, birds are expected to migrate by shorter flight steps, since carrying the heavy fuel loads needed for long non-stop flights comes at considerable cost. Using geolocators, Klaassen et al. (2011) found that Great Snipes (Gallinago media) make long and fast non-stop flights (4300–6800 km in 48–96 hours), not only over deserts and seas but also over wide areas of suitable habitats, which represents a previously unknown migration strategy among land birds. Furthermore, Great Snipes achieved very high ground speeds (15–27 m s−1), which was not an effect of strong tailwind support, and no other animal is known to travels this rapidly over such a long distance. These results demonstrate that some migratory birds are prepared to accept extreme costs of strenuous exercise and large fuel loads, even when stopover sites are available along the route and there is little tailwind assistance. A strategy of storing a lot of energy before departure, even if migration is over benign habitats, may be advantageous owing to differential conditions of fuel deposition, predation or infection risk along the migration route.
Link:
Great Snipe is the fastest migratory bird ever discovered

Great Snipe with geolocator (Photo by Raymond Klaassen)
Several lines of evidence suggest that many birds have an innate capacity to migrate. For example, several non-migratory, resident species of birds, including Stonechats (Saxicola torquata; Helm and Gwinner 2006), Silvereyes (Zosterops lateralis; Chan 1994), and White-crowned Sparrows (Zonotrichia leucophrys; Smith et al. 1969), have been found to exhibit migratory restlessness (or zugunruhe). In addition, recent comparative studies indicate that migratory behavior has evolved repeatedly and very rapidly in different avian lineages (Helbig 2003, Outlaw et al. 2003, Joseph 2005, Davis et al. 2006, Outlaw and Voelker 2006). As an example of how rapidly migratory behavior can develop, House Finches (Carpodacus mexicanus) from a largely sedentary population in southern California were introduced on Long Island, New York, in about 1940 and, by the early 1960s, many of these eastern House Finches had become migratory (Able and Belthoff 1998).
Based on such observations and studies, some investigators have suggested that migration has been a common and widespread characteristic of birds for many millions of years and, if so, then many present-day birds may have inherited the capacity to migrate from their ancestors (Berthold 1999). In other words, given the proper environmental triggers, this innate migratory program (i.e., ‘migratory syndrome’) is activated and allows populations or species of birds to rapidly become migratory. Although there is disagreement about the existence of this migratory syndrome (e.g., Piersma et al. 2005), available evidence seems to suggest that most, if not all, birds have the innate potential to migrate (Salewski and Bruderer 2007). If true, what is the source of this potential?
Unfortunately, as pointed out by Zink (2002), reconstructing the environments where birds evolved and determining when birds first migrated is not currently possible. However, it is reasonable to conclude, as also suggested by Steadman (2005), that migratory behavior existed early in avian history. Thus, for millions of years, natural selection has acted upon some or all of the physiological and behavioral components important in avian migration, maintaining a diversity of migratory behaviors, including, often, its suppression (i.e., non-migratory behavior). However, by removal of the suppression of those genes controlling the various components of migration, migratory behavior can quickly re-appear in a population or species. Among living birds, then, the expression of migratory activity is subject to selective pressures, with those pressures determining if birds are sedentary, short-distance migrants, or long-distance migrants. It is always the case that additional study can alter current ideas or hypotheses. However, available evidence does seem to support the migratory syndrome hypothesis and that means that, when examining present-day birds, the focus must shift from explaining the actual ‘evolution’ of migration (because migratory behavior likely does not evolve de novo) to trying to determine what factors or selective pressures currently acting on birds have contributed to their current migratory, or non-migratory, behavior.
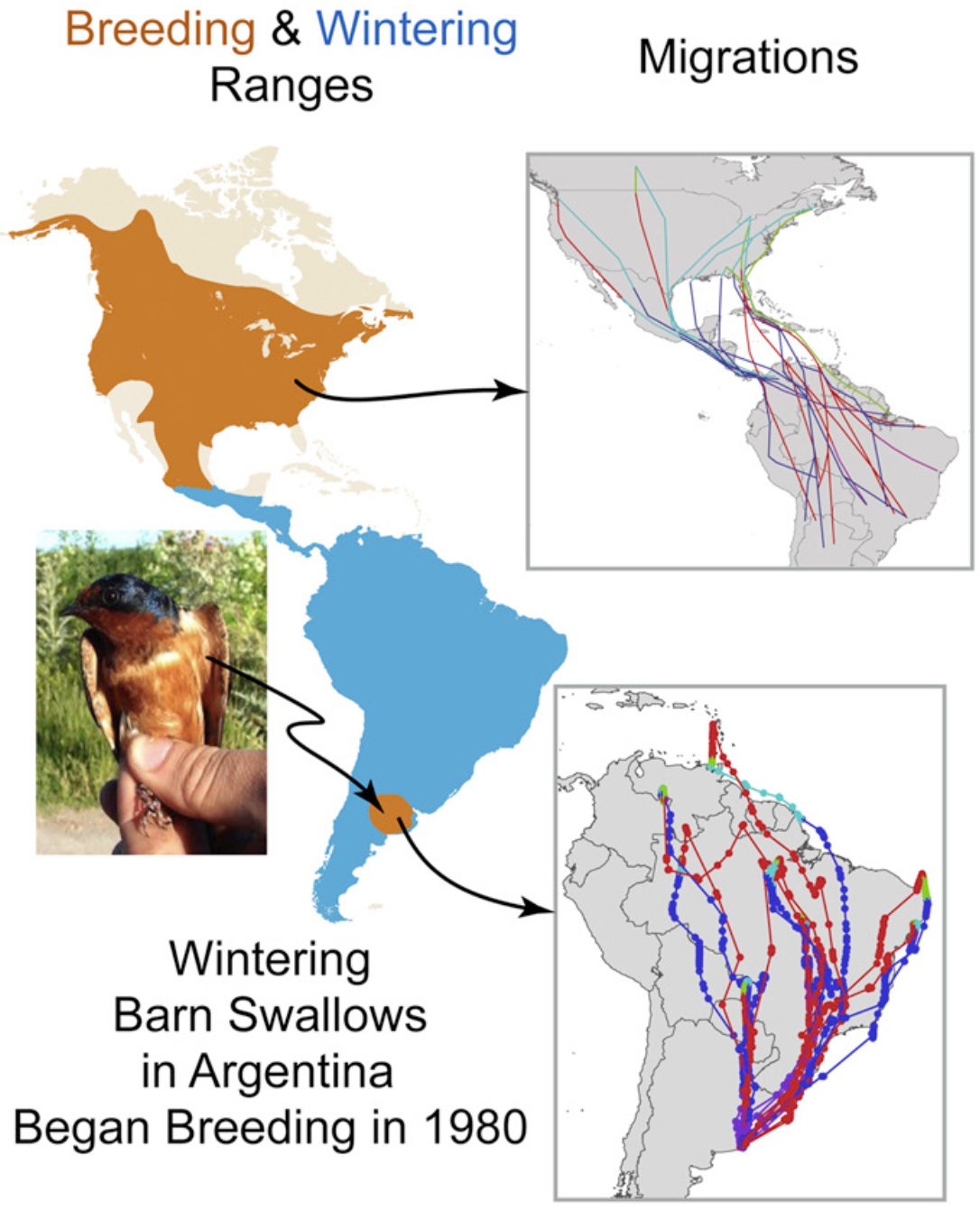
Reconstructed migratory paths of Barn Swallows breeding in North America and a population
now breeding in Argentina. For the North American population the color blue = migration to
breeding areas and red=migration to wintering areas; For the Argentine population the color
red = migration to breeding areas and blue = migration to wintering areas.
Barn Swallows (Hirundo rustica) are globally distributed passerines. Breeding populations in North America generally migrate to wintering sites in South America. However, some Barn Swallow expanded their breeding range an exceptional 7,000 km and, in the early 1980's, began breeding in their regular wintering range in Argentina. Trans-hemispheric breeding attempts have occurred previously in related species of swallows, but only this colonization has persisted. Comparative studies of birds show a remarkable diversity in patterns of change in migratory habits, and these Argentine-breeding swallows might retain ancestral patterns, breeding in Argentina but returning to North America for the austral winter. However, feather isotopes from these birds indicated that they migrate no farther than northern South America. This was confirmed by the results of a solar geolocator study. Thus, this relatively new population of Barn Swallows breeding in Argentina have rapidly changed their movements to migrate no farther north in austral winter than northern South America. In addition, these Argentine Barn Swallows are now following a migratory pattern and choice of wintering areas that is very much like that of other migrant birds from the temperate zone of South America. These Barn Swallows provide evidence that migration is flexibly responsive to an array of environmental cues, making it able to change without the need for underlying genetic change. The rapid change in migration observed in the Argentine Barn Swallows is likely due to phenotypic changes that did not require large changes in the frequencies of associated genes (From: Winkler et al. 2017).
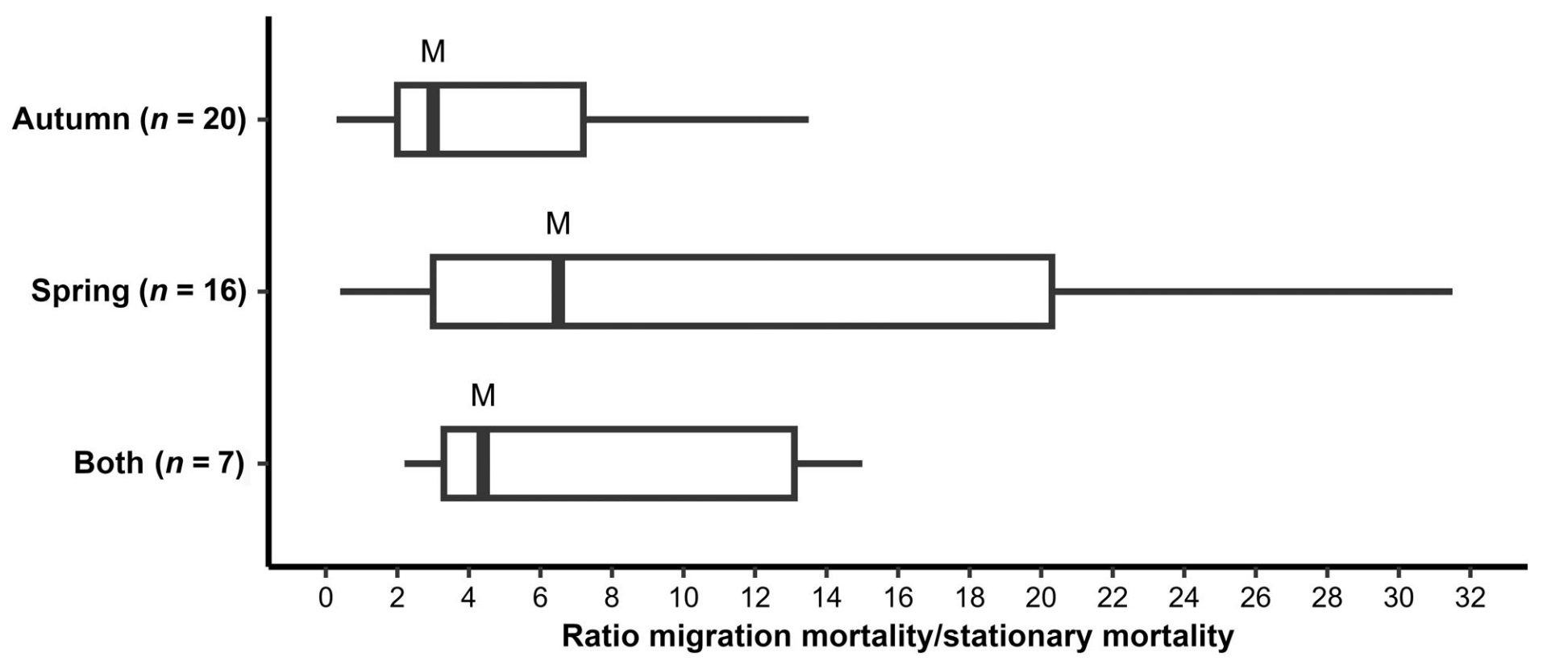
Ratio of migration mortality/stationary period mortality per unit time in different studies, as measured during autumn migration (n = 20 studies),
spring migration (n = 16), or both migrations combined (n = 7). M = median value, blocks indicate 25% of studies above and below the median, and
lines show the full range of values.
Migration mortality in birds. Bird migration is one of the greatest wildlife spectacles, producing massive global changes in the distributions of birds twice each year. To understand the evolution of this phenomenon, it is important to know the costs of these journeys in terms of the mortality they impose. The use of mark/re-sighting and tracking studies has now made it possible, for some bird species, to separate mortality during migration from mortality during stationary periods. This paper aims to assess this information, based mainly on 31 published studies, most of which concern long-distance migrations of passerines, large waterfowl and raptors. Most of these studies revealed that mortality rates were greater during migration than at other times – in some species more than 20 times greater. Overall, on the basis of median values, mortality per unit time during autumn journeys was about 3.0 times greater than mortality during stationary periods, during spring journeys about 6.3 times greater, and during autumn and spring journeys combined 4.4 times greater. The greater overall mortality on spring journeys was largely associated with more adverse wind conditions in spring than in autumn. High mortality rates were especially evident in birds crossing large ecological barriers, such as the Sahara Desert or the Gulf of Mexico, and were higher in that part of their journey than when crossing more benign terrain. There was no increase in mortality during migration in the adults of some long-lived species with high annual survival and predominantly overland journeys; for these birds, much larger samples of year-round tracked individuals will be needed to reveal any seasonal variations in mortality. Within certain species, birds that travelled long distances experienced greater mortality over the journey than those that travelled short distances, but in other species no such relationship was found. In species in which adults and juveniles were followed over the same journey, juveniles showed greater mortality. To judge from other studies, this difference could be attributed to the inexperience of juveniles, their lower feeding rates and flight efficiency, greater vulnerability to hazards such as weather and predation, or more frequent navigational errors. Broadly speaking, the risks of migration vary with features of the birds themselves, with the terrain to be crossed and with weather at the time. It may be assumed that migration persists in the long term because the costs (in terms of associated mortality) are more than offset by the benefits of breeding and wintering in different areas (in terms of improved overall survival and breeding success) (From: Newton 2025).
Migratory and sedentary behaviors of present-day birds
Although more than half of all bird species can be considered migratory, nearly as many species are non-migratory. Migrating is costly in terms of energy and many birds die during migration. For example, Strandberg et al. (2010) used satellite telemetry to monitor four species of raptors crossing the Sahara Desert while attempting to migrate from Europe to Africa and found that 31% of all juveniles and 2% of adults died en route. Newton (2007a) summarized previously documented cases of bird mortality during migration and examples include more than 10,000 Magnolia Warblers (Dendroica magnolia) and other warblers killed in a rainstorm off the Texas coast in 1981, more than 20,000 songbirds killed as a result of dense fog off the coast of Sweden in 1998, and an estimated 200,000 jays, thrushes, and warblers killed during a rainstorm over Lake Manitoba. For migratory species, the benefits of migrating must exceed these costs and, therefore, any explanation for the evolution of migration requires identification of those benefits.
As already noted, birds that migrate vary in the extent to which they migrate (e.g., all vs. part of the population) and in the distances traveled. With such variation, and given that thousands of different species of birds migrate, it is almost certainly the case that different selective factors influence the migratory behavior in present-day birds. However, some factors are likely more important than others, and those most commonly linked to the evolution of migration include seasonal variation in food availability, direct climatic effects on physiological condition, and the risk of nest predation (Fretwell 1980, Cox 1985, Alerstam 1990, Berthold 2001). Variation in food availability could favor migration by allowing exploitation of a seasonal peak in availability during the breeding season, forcing movement out of unproductive areas, or both. Arctic Terns (Sterna paradisaea), with the longest migration route of any bird (about 40,000 km; Hatch 2002), represents an extreme example of this. These terns migrate between maximally productive areas (in terms of daily solar energy reaching the earth’s surface), breeding in productive latitudes in the northern hemisphere and wintering in equally productive latitudes in the southern hemisphere (Figure 5). Climate could contribute to the evolution of migration if seasonal variation in temperatures affected breeding success or survival rates. Migration would also be selected for if risk of nest failure due to predation varied predictably with location, e.g., with latitude or altitude. Of course, these factors are not mutually exclusive and each, to varying degrees, might contribute to the evolution of migratory behavior (Boyle and Conway 2007). In addition, other factors, such as habitat, could also play a role in the evolution of migration.
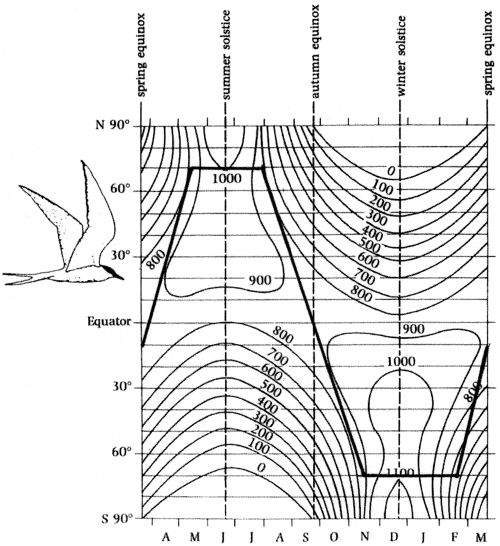
Figure 5. Daily solar energy (cal cm −2) reaching the Earth at different latitudes and times of the year.
The dark line shows the migratory pathway of Arctic Terns through this ‘energy landscape’ between high latitudes in
the northern hemisphere where they breed and those in the southern hemisphere where they molt and spend part of the
non-breeding season (From: Alerstam et al. 2003).

Flight tracks of 11 Arctic Terns tracked from breeding colonies in Greenland (N = 10 birds) and Iceland (N = 1 bird).
Green = autumn (postbreeding) migration (August–November), red = winter range (December–March), and
yellow = spring (return) migration (April–May). Two southbound migration routes were adopted in the South Atlantic,
either (A) West African coast (N = 7 birds) or (B) Brazilian coast
Long-distance migration of Arctic Terns -- The annual migration of Arctic Terns (Sterna paradisaea) from boreal and high Arctic breeding grounds to the Southern Ocean is likely the longest seasonal movement of any animal. Egevang et al. (2010) tracked 11 Arctic Terns fitted with miniature (1.4-g) geolocators and found that some individuals travelled more than 80,000 km annually. At the end of the breeding season, tagged birds traveled southwest to a stopover region of deep water in the eastern portion of the Newfoundland Basin and the western slope of the mid-North Atlantic Ridge where they remained for an average of about 25 days. Between 5 and 22 September, all 11 birds continued their migration southeast toward the West African coast. South of the Cape Verde Islands (~10° N), however, migration routes diverged: seven birds continued to fly south parallel to the African coast, whereas four others crossed the Atlantic to follow the east coast of Brazil. Birds in both groups ceased their directed southbound transits at ~38–40° S, and shifted to a pattern of predominantly east–west movements. All birds subsequently moved south, spending the austral summer (December–March) in the Atlantic sector of the Southern Ocean. This region is particularly productive, and supports higher densities of a key prey for many seabirds (Antarctic krill, Euphausia superba) than elsewhere in the Southern Ocean. All birds began the return migration to breeding colonies in early–mid April, always traveling over deep water at considerable distance from continental shelf margins. The average annual distance traveled, from departing the breeding site in August to return in late May/early June (i.e., excluding movements within the breeding season) was 70,900 km (range =59,500–81,600 km).
Arctic Tern migration
Levey and Stiles (1992) proposed that bird migration between the Nearctic and Neotropics arose from the tendency of some species to move in response to changes in resource abundance. Specifically, they noted that many short-distance Neotropical migrants are primarily frugivorous and occupy habitats, including open, non-forested areas, forest edge, and the forest canopy, that exhibit greater fluctuation in temperature and humidity than forest-interior habitat. Fluctuating conditions cause fluctuation in resource availability that favors species that migrate. Over time, some birds in lineages dependent on these ‘fluctuating’ habitats and resources and with pre-existing tendencies to migrate moved longer distances and became long-distance migrants. Based on this ‘evolutionary precursor’ hypothesis, habitat characteristics contribute to variation in resource availability that favors the evolution of migratory behavior.
In a comparative study involving 379 species in the suborder Tyranni (flycatchers, manikins, cotingas, tityras, and becards), Boyle and Conway (2007) examined the possible relationships between habitat, diet, behavior, and migration more broadly, considering both sedentary and migratory species, birds with a wider variety of food habits (frugivores and insectivores) and social organization (solitary vs. flocking species), and birds occupying a wider variety of habitats (ground/thickets, forest understory, forest midstory, canopy, open/arid, and disturbed). Their analysis revealed that habitat and diet influence the tendency of birds to migrate, but in rather complex ways (Figure 6). Species found in thickets and forest canopy and understory were more likely to migrate if frugivorous, but species in open/arid and disturbed habitats were more likely to migrate if insectivorous. Without better information concerning the extent to which food resources vary in different habitats, interpreting these results is difficult. However, one possibility is simply that, regardless of habitat, species depending on food resources that exhibit seasonal variation in availability are more likely to evolve migratory behavior than species depending on less seasonal resources (resource availability hypothesis; Boyle and Conway 2007).
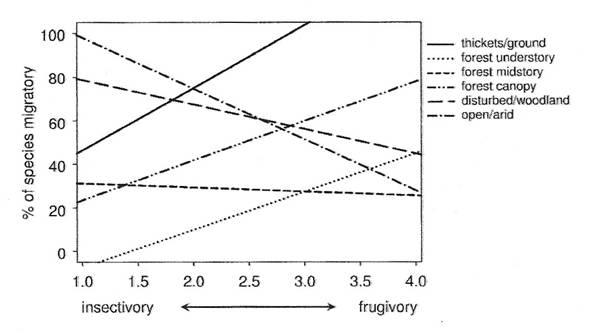
Figure 6. Percentage of species in the Tyranni that migrate varies with diet (highly insectivorous to highly frugivorous) and
habitat use. The six lines illustrate how diet and habitat interact; birds of thickets, forest understory, and canopy are more likely
to be migratory if they are frugivorous. In contrast, increasing frugivory is associated with a decreasing likelihood of being migratory for
birds of disturbed and arid habitats. Boyle and Conway (2007) plotted linear regression lines for each habitat category based on the
proportion of species that migrate for each level of diet along a scale from highly insectivorous (1.0) to highly frugivorous (4.0).
In addition to resource availability, another factor that appears to influence migratory behavior is the size of foraging groups. Boyle and Conway (2007) found that species of birds that are solitary foragers are much more likely to be migratory than species that forage in pairs or groups. A likely explanation for this relationship between foraging behavior and tendency to migrate is that foraging with conspecifics likely improves foraging efficiency. If so, then migration and foraging with conspecifics may simply represent two alternative strategies for coping with seasonal variation in resources, either leave or forage with others to increase foraging efficiency.
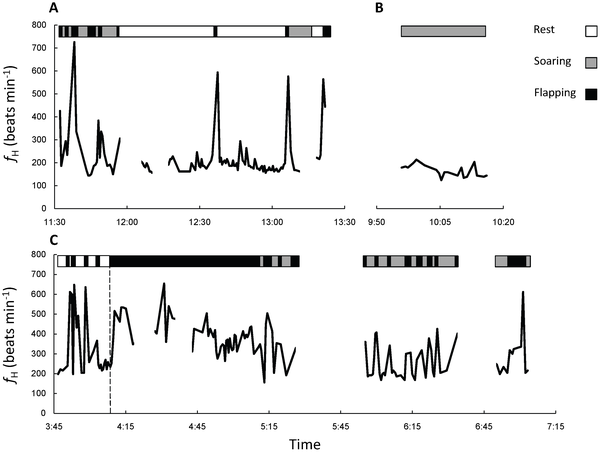
Heart beat frequency (fH) traces of two European Bee-eaters engaged in different activities during stopover and cross-country flight.
Soaring-gliding flight by small birds during migration -- Many avian species soar and glide over land. Evidence from large birds (>0.9 kg) suggests that soaring-gliding is considerably cheaper in terms of energy than flapping flight, and costs about two to three times the basal metabolic rate (BMR). Yet, soaring-gliding is considered unfavorable for small birds because migration speed in small birds during soaring-gliding is believed to be lower than that of flapping flight. Nevertheless, several small bird species routinely soar and glide. To estimate the energetic cost of soaring-gliding flight in small birds, Sapir et al. (2010) measured heart beat frequencies of free-ranging migrating European Bee-eaters (Merops apiaster, ~55 g) using radio telemetry, and established the relationship between heart beat frequency and metabolic rate (by indirect calorimetry) in the laboratory. Heart beat frequency during sustained soaring-gliding was 2.2 to 2.5 times lower than during flapping flight, but similar to, and not significantly different from, that measured in resting birds. Soaring-gliding metabolic rate of European Bee-eaters was estimated to be about twice their basal metabolic rate (BMR), which is similar to the value estimated in the Black-browed Albatross (Thalassarche melanophrys, ~4 kg). Soaring-gliding migration speed was not significantly different from flapping migration speed, and there was no evidence that soaring-gliding speed was slower than flapping flight in bee-eaters, contradicting earlier estimates that implied a migration speed penalty for using soaring-gliding rather than flapping flight. Small birds may soar and glide during migration, breeding, dispersal, and other stages in their annual cycle because it may entail a low energy cost of transport. The energy cost of soaring-gliding may be proportional to BMR regardless of bird size, as theoretically deduced by earlier studies.

European Bee-eater (Photo credit: Jorge Rodrigues)
Migratory birds exhibit great variation in the distance of their migratory journeys, with some short-distance migrants moving just a few hundred kilometers and some long-distance migrants traveling several thousand kilometers (Figure 7). Several factors can contribute to this variation in migration distance among different species and populations. For example, among birds in the suborder Tyranni, migration distance appears to be influenced by food habits, with insectivorous species tending to migrate longer distances than frugivorous species (Boyle and Conway 2007). Similarly, analysis of the relationship between migration distance and food habits among songbirds of the western Palearctic revealed that insectivorous species tend to migrate greater distances than granivorous (seed-eating) birds or those that fed on both seeds and insects (Figure 8; Newton 1995, 2003b). Year-round insectivory is likely a consequence rather than the cause of long-distance migration in birds (Boyle and Conway 2007). That is, during the breeding season, fruit and seeds are not as abundant at high latitudes as insects and, therefore, fewer frugivorous and granivorous birds breed there. In addition, during the non-breeding season, fruit and seeds are likely more abundant than insects at mid-latitudes. As a result of the availability of prey, therefore, many insectivorous species breed at higher latitudes and migrate relatively long distances to winter at low latitudes.
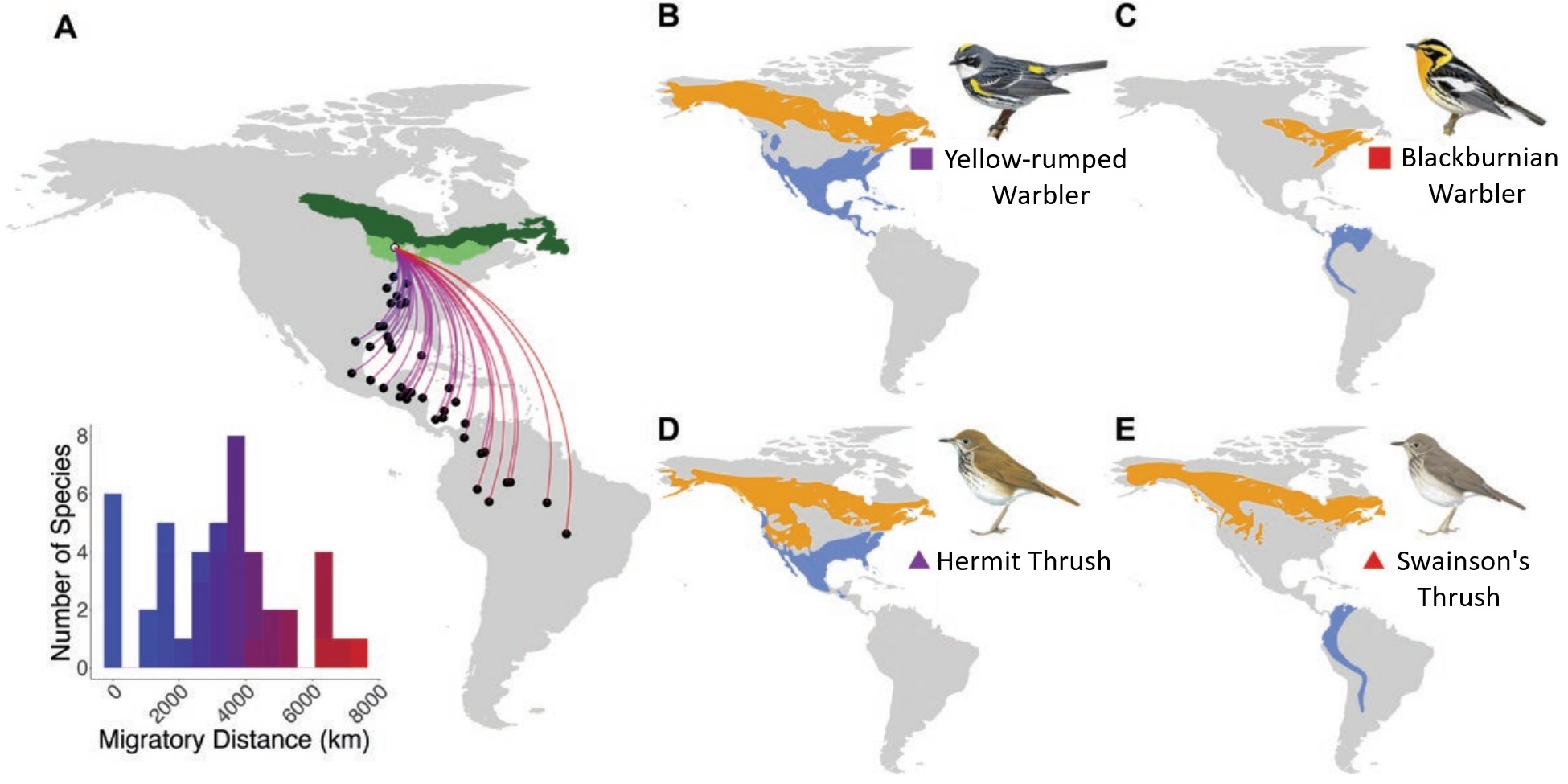
(A) Many species of birds breed in the North
American boreal forest belt, but winter in disparate locations. Dark green
shading shows the Boreal Softwood Shield, and light green
shading the Boreal Hardwood Transition. Approximate
migration distances are indicated for 45 species that breed in the boreal forest belt. (B-E) Examples of species representing
short-distance (B and D) and long-distance
(C and E) migratory species pairs in two genera. The squares (Setophaga)
and triangles (Catharus) are colored according to migratory distance in (A).
Seasonal migration is intrinsically connected to the balance of survival and reproduction, but whether migratory behavior influences a species position on the slow-fast continuum of life history is poorly understood. The relationship between migration distance and annual survival could be mediated by several mechanisms, including differing conditions experienced on the wintering grounds. Based on a study of 45 species of birds (41 passerine species from 11 families and 4 woodpecker species), Winger and Pegan (2021) found that boreal-breeding birds that migrate long distances have higher annual adult survival and lower annual reproductive investment than boreal species that migrate shorter distances to winter closer to their breeding areas. After controlling for body size and phylogeny, these authors found that migration distance and apparent annual adult survival are positively related across species. Both migration distance and survival are positively correlated with wintering in environments that are warmer, wetter, and greener. However, these longer migrations are associated with reduced time spent in the breeding areas, smaller clutch sizes, and lower fecundity (clutch size × maximum number of broods per year). Although seasonal migration is often associated with high mortality, these results suggest that long-distance migration imposes selection pressures that both confer and require high adult survival rates. That is, because of its reproductive cost, long-distance migration can only persist if balanced by high adult survival. For boreal birds, the evolution of the longest migrations results in the highest survival, but at an inherent cost to annual (but not necessarily lifetime) fecundity.
Among raptors, food habits can also influence migration distance. For example, among raptors of the western Palearctic, those that prey on endotherms (or warm-blooded prey; i.e., birds and mammals) tend to migrate shorter distances than those that prey on either ectotherms (or cold-blooded prey; i.e., insects, amphibians, and reptiles) or both ectotherms and endotherms (Figure 9; Newton 2003a). Again, this difference is a result of food availability, raptors that breed at higher latitudes and tend to prey on ectotherms must migrate further to wintering areas because their primary prey may not be available at high- to mid-latitudes during the non-breeding period.

Figure 7. White-rumped Sandpipers breed in the tundra of northern Canada and Alaska and winter
in southern South America, a journey of up to 4000 km (Source: Parmalee 1992).

Figure 8. Migration distance relative to breeding location (latitude) and diet for songbirds of the western Palearctic.
Lines were calculated by linear regression analysis based on data for individual species (Newton 1995, 2003b).

Figure 9. Migration distances of western Palearctic raptors relative to breeding latitude and diet.
Lines calculated by linear regression analyses (From: Newton 2003a).
Although food habits can clearly influence migration distance, for many populations and species of birds, other factors can also be important. Among shorebirds that breed at high latitudes in North America, the risk of nest predation decreases with increasing latitude (McKinnon et al. 2010, Figure 10). As a result, natural selection may favor individuals that migrate further and nest at higher latitudes. Of course, migrating further north also entails a greater energetic cost and the need to withstand the harsher environmental conditions. Thus, although a strategy of migrating further to reduce the risk of nest predation would appear to be beneficial, it remains to be determined whether the benefits of nesting further north exceed the costs (McKinnon et al. 2010).
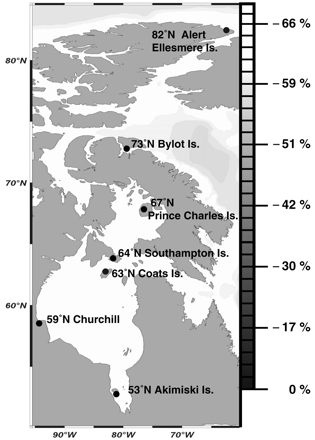
Figure 10. Average latitudinal decrease in nest predation risk and map of the shorebird
breeding sites where artificial nests were monitored. The decrease in predation risk (3.6% per degree
relative to the southernmost site, Akimiski Island) is indicated at 5° intervals on the latitudinal scale at right (From: McKinnon et al. 2010).
Migration distances can also be influenced by the presence of ecological or geographical barriers, such as large bodies of water, deserts, and mountain ranges. In most cases, the distance across these barriers is within the birds’ potential flight range capacity and, in many cases, some, if not most, birds do cross them. For example, numerous Neotropical migrants cross the Gulf of Mexico during migration to and from breeding and wintering areas in North American and Central and South America. Similarly, many birds migrating between Europe and sub-Sahara Africa cross the Mediterranean Sea and the Sahara Desert.
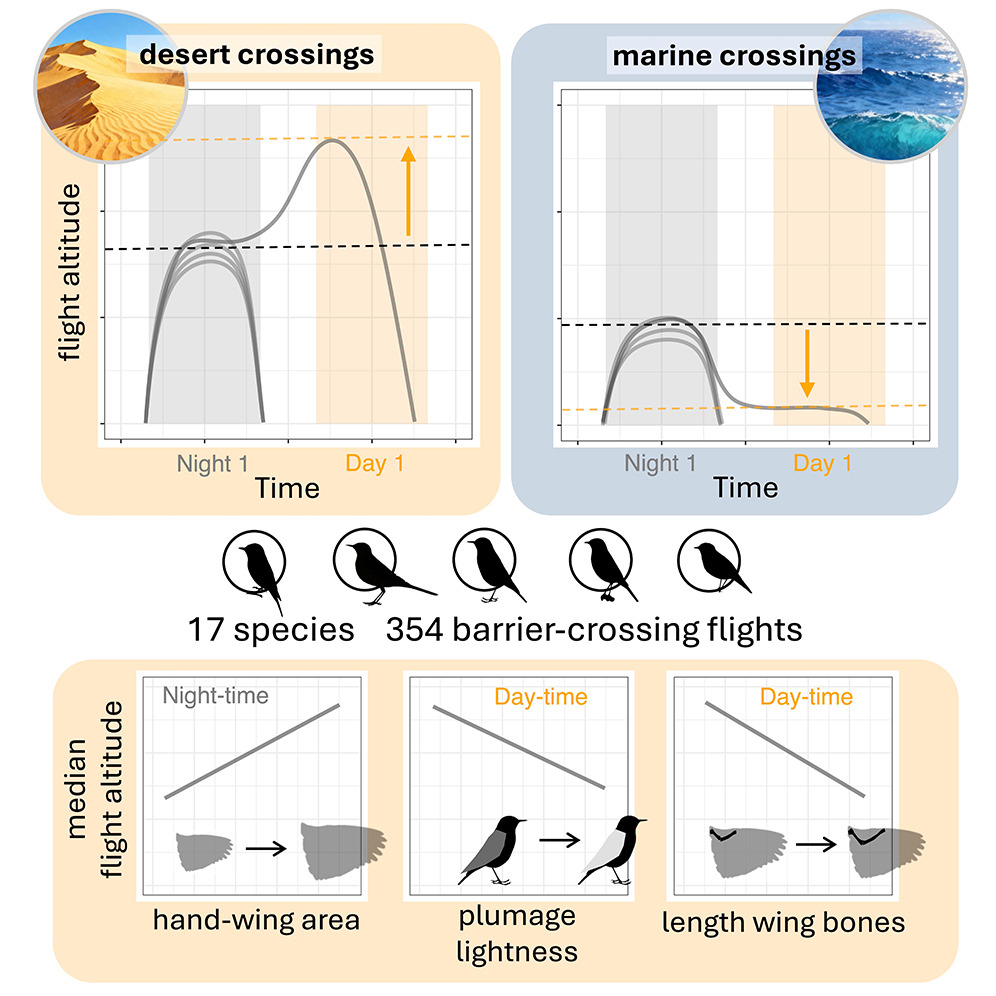
Using data from 67 recovered multi-sensor loggers from 17 species of birds, Dufour et al. (2025) examined how
small landbirds crossed two major marine barriers (Bay of Biscay, Mediterranean Sea) and the Sahara Desert.
The investigators used a comparative approach to test the influence of wing morphology and plumage color on flight altitude.
Birds exhibited important differences when crossing barriers: over the desert, they averaged 1,600 m altitude at night and
2,800 m altitude during daytime flights. During marine crossings, they flew at lower altitudes (mean = 750 m), and sometimes
just above water. Flight altitude increased with wing area, perhaps because larger winged birds can generate more lift with less
effort,
and species with darker plumage flew higher over the Sahara, likely to enhance heat dissipation and reduce solar heating. Birds may fly
at lower altitudes over water to save energy, particularly with headwinds or crosswinds, because wind velocities may be lower closer
to the water's surface (From: Dufour et al. 2025).
Other birds use longer migration routes to avoid crossing the barriers (Figures 11 and 12). For some migrating birds, some barriers simply cannot be crossed. For example, hawks, such as Broad-winged Hawks (Buteo platypterus) that rely on soaring flight to travel long distances are dependent on rising air generated by thermals and, therefore, avoid migration routes that would take them over large bodies of water (Figure 13). For those birds physiologically capable of crossing barriers, numerous factors likely influence the decision to either cross or avoid barriers located along their migration routes, including their physical condition (e.g., fat stores), weather conditions, wind speed and direction, and risk of predation (Alerstam 2001).

Figure 11. Detour migration by Brent Geese (left map, a) and Common Eiders (right map, b. Departure and destination
locations are indicated by small circles. The shortest distance migration routes are shown as straight lines; curved lines indicate
the actual migration routes (From: Alerstam 2001).
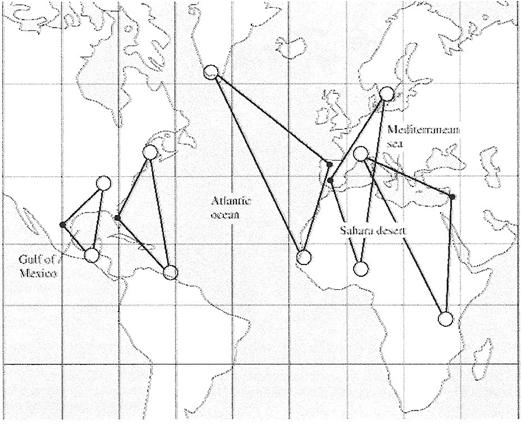
Figure 12. Examples of observed and potential detours in bird migration at ecological barriers like the Mediterranean Sea,
Sahara Desert, Atlantic Ocean, and Gulf of Mexico. Shortest routes are indicated by straight lines between large open circles.
Longer ‘detour’ routes taken by some migrants are indicated by lines between connecting large open circles via the small filled circles
(From: Alerstam 2001).

Figure 13. Migration routes of Broad-winged Hawks between breeding areas in Minnesota and Maryland and
wintering areas in Central and South America. For hawks migrating from Maryland in the eastern United States, a direct
route across the Gulf of Mexico would be much shorter, but there are no thermals over open water so they take the
longer, land-based route. Data were collected by satellite telemetry (From: Haines et al. 2003).
Migratory shorebirds
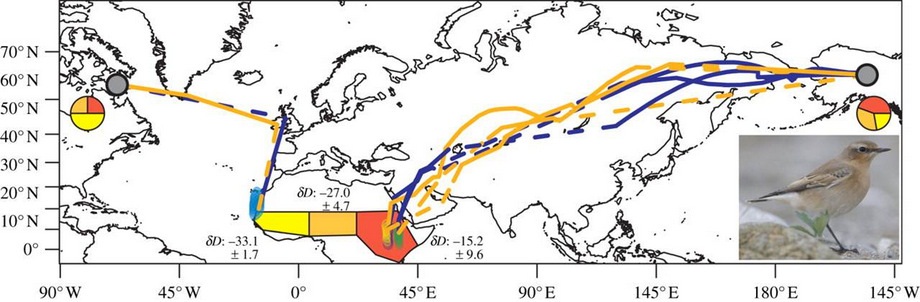
Migration routes and wintering grounds of three Northern Wheatears breeding in Alaskan (AK) and one in the eastern Canadian Arctic (CN; grey dot, breeding area, blue, autumn migration, orange, spring migration, dashed lines indicate uncertainty in migration routes close to equinoxes). Fifty per cent kernel densities of winter fixes (beginning of December 2009–end of February; purple, bird AK-1; green, bird AK-2; orange, bird AK-3; blue, bird CN-1) are given). Pie charts indicate the proportion of individuals (AK: n = 9, CN: n = 4) originating from one of the three pre-defined wintering regions (red, western; orange, central; yellow, eastern) [8] based on stable-hydrogen isotope (δD) values in winter grown feathers and the δD values within each wintering region (mean ± s.d. shown).
Cross-hemisphere migration
Link:
A songbird's epic migration across hemispheres
Male Northern Wheatear
(Photo source: Wikipedia)
Differential and partial migration
Among some species of migratory birds, all individuals in a population or species may share the same general breeding and wintering areas, with no spatial separation of adults and juveniles or males and females. However, for other species, particularly among short- and medium-distance migrants, wintering areas may vary with sex, age, or both. Some individuals may migrate whereas others do not (partial migration) or some individuals migrate greater distances than others (differential migration; Figure 14).
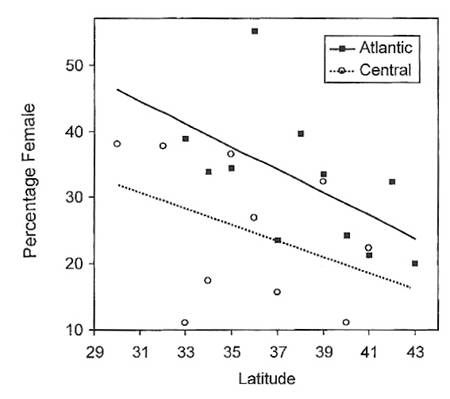
Figure 14. Relationship between latitude and percentage of females in the wintering populations of
White-throated Sparrows in the eastern (Atlantic) and central regions of the United States. Sex ratios are
more biased towards females at more southern latitudes, indicating that females tend
to winter further south than males (From: Jenkins and Cristol 2002).
Among partial and differential migrants, a number of factors can potentially influence either a bird’s decision to migrate or not or how far to migrate, including age, sex, physical condition, size, and dominance status. In addition, the migratory behavior of partial migrants can either be obligate, i.e., genetically (innately) fixed at the individual level (Lundberg 1988), or facultative, with migratory decisions based on conditions that can change over time (Ketterson and Nolan 1983).
Several hypotheses have been proposed to explain partial and differential migration. The Arrival Time hypothesis proposes that the individuals that establish breeding territories are less likely to migrate or, if they migrate, to migrate shorter distances because remaining in or near breeding areas makes it more likely that they will be able to acquire (or reacquire) high-quality territories (King et al. 1965). The Dominance hypothesis suggests that migratory decisions are based on dominance status, with subordinate individuals in a population more likely to migrate because, if they stay or migrate shorter distances, dominant individuals are likely to out-compete them for access to needed resources (Gauthreaux 1982). The Body Size hypothesis posits that larger individuals with smaller surface area-to-volume ratios are less likely to migrate or to migrate long distances because they are better able to withstand colder temperatures and food shortages (Ketterson and Nolan 1976). These hypotheses are all based on the results of studies conducted in north temperate areas where there can be extreme seasonal differences in environmental conditions (e.g. temperature and day length) and food availability. In addition, all three are based on the assumption that staying further north can be costly due to adverse weather conditions, but can also be beneficial because of shorter migration distances. Testing these hypotheses is often difficult because, in many species of birds, males are larger, dominant, and establish breeding territories. In such species, all three hypotheses lead to the same prediction: larger, dominant males should winter further north. These hypotheses are also not mutually exclusive; multiple factors can contribute to the evolution of partial migration.
As an example of the difficulty in differentiating among these hypotheses, White-throated Sparrows (Zonotrichia albicollis) breed across most of eastern Canada and the northeastern United States and, during the non-breeding season, migrate as far south as the Gulf of Mexico. These sparrows exhibit differential migration, with males tending to winter further north than females (Figure 12). Male White-throated Sparrows are larger than and dominant to females (Piper and Wiley 1989), and arrive in breeding areas one to two weeks earlier than females to establish territories (Falls and Kopachena 2010). Thus, any or all of the proposed hypotheses (Arrival Time, Dominance, or Body Size hypotheses) could explain differential migration by White-throated Sparrows.
Some species, however, have characteristics that make them suitable for testing these hypotheses, and studies have revealed interspecific differences in the factors that have led to the evolution of partial migration. For example, House Finches (Carpodacus mexicanus) in the eastern United States exhibit differential migration, with males tending to winter further north than females. This difference appears to be best explained by the Body Size hypothesis (Belthoff and Gauthreaux 1991) because male House Finches do not defend territories (and so, based on the Arrival Time hypothesis, they have no need to winter closer to breeding areas) and females are typically dominant to males (so, based on the Dominance hypothesis, females should winter further north). However, male House Finches are larger than females and, as predicted by the Body Size hypothesis, should winter further north because they can better cope with colder temperatures and reduced food availability.
Partial migration by Lesser Black-backed Gulls (Larus fuscus) appears to be best explained by the Arrival Time hypothesis (Marques et al. 2010). Older Black-backed Gulls tend to winter further north, closer to breeding areas, than younger gulls. These gulls exhibit minimal variation in body size so the Body Size hypothesis cannot explain the age-related difference in migration distance. The Dominance hypothesis predicts that dominant gulls (those 4 or more years old) should winter closest to the breeding grounds. However, three-year old gulls that will be breeding for the first time winter as close or even closer to breeding areas than many older, more dominant gulls, suggesting that begin close and arriving early in breeding areas best explains the winter distribution of Black-backed Gulls (Marques et al. 2010).
Few studies have provided support for the Dominance hypothesis. However, Kjellén (1994) found that, in several species of raptors, juveniles were more likely to migrate than adults and, in addition, females were less likely to migrate than males. Adult raptors are dominant to juveniles and exhibit reversed sexual dimorphism, with females larger than and dominant over males. These results, therefore, support the Dominance hypothesis, with dominant adults and larger, more dominant females tending to winter further north.
Few investigators have examined partial migration in the tropics where wet-dry cycles predominate. However, a recent study of Tropical Kingbirds (Tyrannus melancholicus) provided support for the Food Limitation hypothesis (Jahn et al. 2010). This hypothesis predicts that, among insectivorous species, larger individuals with greater energetic needs are more likely to migrate to wetter areas to find sufficient food. In contrast to the other three hypotheses where larger individuals, generally males, are predicted to be less likely to migrate, Jahn et al. (2010) found that the largest male Tropical Kingbirds that were typically older and dominant over younger individuals were most likely to migrate from breeding areas. Because Tropical Kingbirds feed on flying insects and never forage in flocks, dominance status has less effect on their ability to access resources (compared to many granivores and omnivores that feed in flocks). What is more important is the abundance of flying insects. When insect availability drops during the dry season (coinciding with the non-breeding period), larger males may be unable to meet their energetic needs and must migrate to wetter areas with more insects. In contrast, smaller individuals require less energy and fewer insects and need not migrate.

Black-tailed Godwit
(Photo source: Wikipedia)
Is longer distance migration costly? -- For many migratory bird species, the latitudinal range of the winter distribution spans thousands of kilometers, thus encompassing considerable variation in individual migration distances. Pressure to winter near breeding areas is thought to be a strong driver of the evolution of migration patterns, as individuals undertaking a shorter migration are generally considered to benefit from earlier arrival on the breeding grounds. However, the influence of migration distance on timing of arrival is difficult to quantify because of the large scales over which individuals must be tracked. Using a unique dataset of individually-marked Icelandic Black-tailed Godwits (Limosa limosa islandica) tracked throughout the migratory range by a network of hundreds of volunteer observers, Alves et al. (2011) quantified the consequences of migrating different distances for the use of stopover sites and timing of arrival in Iceland. Modelling of potential flight distances and tracking of individuals from across the winter range shows that individuals wintering further from the breeding grounds must undertake a stop-over during spring migration. However, despite travelling twice the distance and undertaking a stop-over, individuals wintering furthest from the breeding grounds are able to overtake their conspecifics on spring migration and arrive earlier in Iceland. Wintering further from the breeding grounds can therefore be advantageous in migratory species, even when this requires the use of stop-over sites which lengthen the migratory journey. As early arrival on breeding sites confers advantages for breeding success, the capacity of longer distance migrants to overtake conspecifics is likely to influence the fitness consequences of individual migration strategies. Variation in the quality of wintering and stopover sites throughout the range can therefore outweigh the benefits of wintering close to the breeding grounds, and may be a primary driver of the evolution of specific migration routes and patterns.
During their annual cycles, many birds in mountainous areas move up and down in altitude, a phenomenon called altitudinal migration. By moving relatively short distances in altitude, birds gain the same climatic benefit as latitudinal migrants that travel hundreds or thousands of kilometers (Newton 2008). Short-distance altitudinal migration is particularly common in tropical regions. For example, on the Atlantic slope of Costa Rica, about 30% of breeding bird species exhibit altitudinal migration and most altitudinal migrants are primarily frugivores or nectarivores (Stiles 1983).
Somveille et al. (2026) found that altitudinal migration is common, with 31.1% of avian populations (n = 10,998 bird populations in 34 mountain regions) living year-round on mountain slopes being altitudinal migrants (i.e., having mean seasonal altitudes separated by more than 200 m). Among altitudinal migrants, few populations (only 96) radically shift their distribution along the elevational gradient (i.e., more than 1000 m on average), mirroring the pattern observed for latitudinal migration where few avian species migrate very long distances (e.g., trans-equatorial migrants). These authors also found that the proportion of altitudinal migrants in a mountain region increased with increasing latitude, supporting the hypothesis that altitudinal migration is an adaptation to seasonality.
A number of factors could potentially influence altitudinal migration. As with latitudinal migration, variation in food availability may be a factor in the altitudinal migration of some species. For example, Spotted Owls (Strix occidentalis) in the Sierra Nevada Mountains of California migrate to lower altitudes during the winter (Laymon 1989), likely because heavy snow at higher altitudes makes locating and capturing their prey (e.g., rodents) more difficult. In Costa Rica, Bare-necked Umbrellabirds (Cephalopterus glabricollis) appear to respond to variation in fruit abundance, breeding at higher elevations during the period of peak fruit abundance and moving to lowland areas where fruit abundance peaks during the non-breeding season (Chaves-Campos et al. 2003). More generally, the results of Somveille et al.'s (2026) analysis suggest that the seasonal distribution of birds in mountains is largely shaped by species minimizing energetic costs while maximizing energy acquisition and that altitudinal migration is a behavioral mechanism allowing birds to optimize their energy budgets in the face of seasonality (as well as competition for access to resources).
The risk of nest predation may also influence distances moved by altitudinal migrants. Using artificial nests, each with two eggs (one infertile canary egg and one egg made of clay), placed along an altitudinal gradient in Costa Rica, Boyle (2008) found that nest predation rates generally declined with increasing altitude (Figure 15). This suggests that, for altitudinal migrants in the tropics, one potentially important factor in determining how high to migrate to breeding sites is predation risk, and some birds may migrate further and higher because of the benefits associated with lower rates of nest predation.

Figure 15. Relationship between elevation and probability of nest predation for 375 nests located at
eight sites ranging in elevation from 40 to 2780 m on the Atlantic slope of Costa Rica. The axis on the
right represents the proportion of nests predated at each site. The line is a regression line showing the linear
relationship between elevation and predation (From: Boyle 2008).
Altitudinal migration may also be an outcome of competition. For example, American Dippers (Cinclus mexicanus) are aquatic songbirds that breed along fast-flowing rivers and mountain streams and feed on freshwater invertebrates. Dippers construct domed nests close to water and prefer sites inaccessible to predators, protected from floods, and with a horizontal ledge or crevice for support (Kingery 1996). Dippers prefer to breed at lower elevations, and those that do so produce more offspring than those that breeding at higher elevations (Gillis et al. 2008). However, the availability of territories with suitable nest sites is limited at lower elevations and most Dippers must either migrate to higher elevations to breed (Gillis et al. 2008, Mackas et al. 2010).
Molting is energetically expensive for birds and altitudinal migration in some species may reflect a strategy of moving to more productive areas at higher elevations after breeding to better meet the energetic and nutritive demands of molt. For example, Cassin's Vireos (Vireo cassinii) breed in low elevation coniferous forests in the Cascade Mountains, but, after breeding, move up-slope at least 300 m to molt in wetter, high-elevation Douglas-fir (Pseudotsuga menziesii ) forests (Rohwer et al. 2008) where insect prey may be more abundant.
Weather can also lead to altitudinal migration. For example, White-ruffed Manakins (Corapipo altera) in Costa Rica migrate to higher altitudes to breed because fruit availability is greater at higher than at lower altitudes, but migration to lower altitudes during the non-breeding period appears to be in response to weather conditions (heavy rains at high altitudes; Boyle 2010, Boyle et al. 2010).
American Dipper
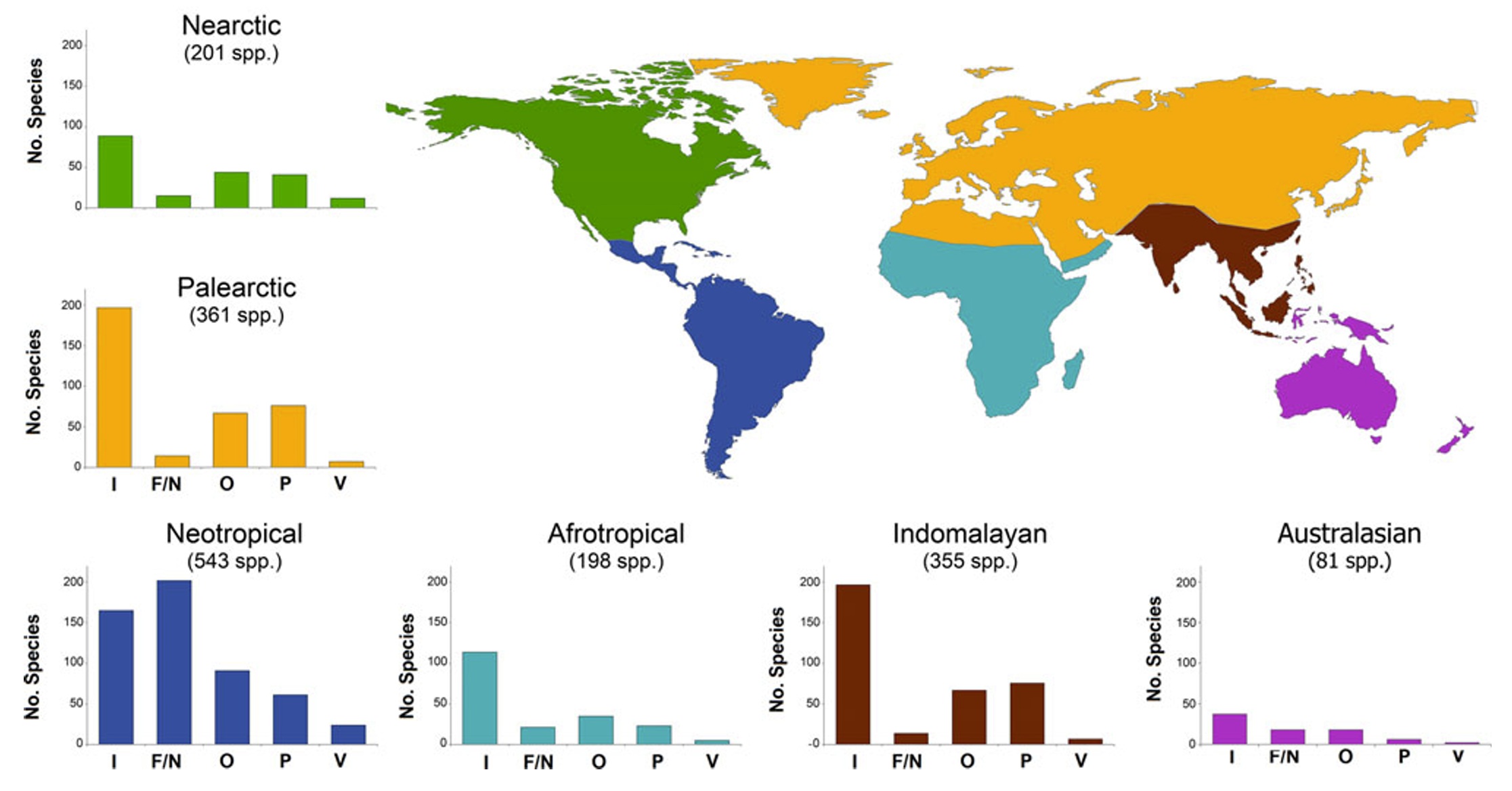
Foraging guilds of altitudinal migrants in different zoogeographic realms. I, invertivore birds; F/N, frugivores/nectarivores; O, omnivores; P, plantivores;
and V, carnivores that consume mainly vertebrates and/or carrion.
Altitudinal migration is the seasonal altitudinal movement of birds from breeding areas to non-breeding or wintering areas at different elevations. Barcante et al. (2017) conducted an extensive bibliographic survey and compiled a list of altitudinal migrant birds worldwide. Species were characterized in terms of their foraging guilds because the spatial distribution of food resources along altitudinal gradients is often evoked as a driver of bird altitudinal migration. The authors identified 1238 species of altitudinal migrants, ~10% of the ~10,000 extant species of birds. Most species of altitudinal migrants were invertivores rather than frugivores or nectarivores. This general pattern held true for all zoogeographic realms except the Neotropics, where nectarivores and frugivores predominated among altitudinal migrants. The prevalence of invertivore birds among altitudinal migrants is not unexpected because this is the most common foraging guild among birds worldwide. Overall, there was no prevalence of any specific foraging guild among altitudinal migrants across zoogeographic regions. The results of studies to date suggest that altitudinal migration by birds may be driven by a number of factors, including access to increased food resources for breeding or molting, weather conditions, and mating and nesting opportunities.
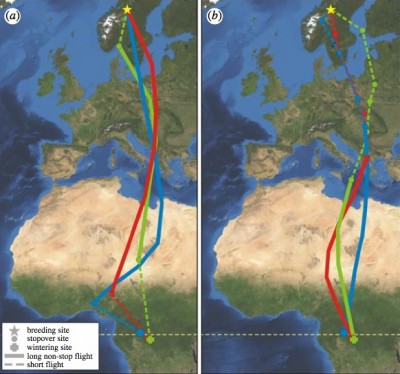
Migrating birds sometimes follow different routes during spring and autumn and, when one route is east or west of the other, this is referred to as loop migration. Loop migration occurs when conditions, typically wind direction, favor different routes in fall and spring. For example, American Golden Plovers (Figure 5), several other species of shorebirds, and even Blackpoll Warblers (Dendroica striata; Figure 16), migrate from the northeastern United States across the Atlantic Ocean to the northeastern coast of South America during the fall. This long flight is made easier, and energetically less expensive, by prevailing winds that help carry birds off the coast of the United States toward the southeast and then, in the mid-Atlantic, winds that help carry birds southwest to the coast of South America (Figure 17). However, during the spring, wind conditions over the Atlantic are no longer favorable (Figure 17) and a northward migration further west and largely over land is the more favorable route.
Figure 16. Blackpoll Warblers migrate over the Atlantic Ocean from the northeastern coast of the United States
to the northeastern coast of South America in the fall, but take a more westerly route over the Gulf of Mexico and the
United States and Canada in the spring (From: Hunt and
Eliason 1999).

Figure 17. Distribution of average main pressure areas and wind patterns
during the fall and winter (above)
and spring and summer (below). Note that wind conditions are favorable for flights from the northeastern
United States to South America over the Atlantic Ocean in the fall (winds generally to the southeast over
the North Atlantic and switching to the southwest and carrying birds toward the northeastern coast of South America
further south). In contrast, wind conditions are not favorable for a return flight over the Atlantic in the spring,
with bird facing a headwind off the northeastern coast of South America (From: Liechti 2006).
Migrating birds rely on stored energy and nutrients to fuel their flights, and many birds, especially small landbirds, cannot store enough energy to fly nonstop between breeding and wintering areas. So, for most birds, migration is divided into alternating periods of flight and stopover, with time at stopover sites spent foraging to deposit fuel for the subsequent flight(s) (Figure 18). The time spent at stopover sites is influenced by a bird’s condition when arriving at a site and by conditions, such as food availability and weather, at the site. The overall speed of migration is greatly influenced by the time spent at stopover sites, and this speed can be of critical importance because it determines when migrants arrive at breeding and wintering sites.
Experiments suggest that stopover duration is short if foraging success is poor and fuel deposition rates are low or negative (Biebach 1985, Yong and Moore 1993). More generally, Schaub et al. (2008) found that birds that accumulated fuel stores at medium rates remained at stopover sites longer than those that either lost fuel stores during their stopover or were able to increase their fuel stores quickly. However, the decision about when to leave a stopover site also appears to be influenced by the location of a site, with birds at sites located just before a large ecological barrier (e.g., a desert) generally stayed long enough to deposit sufficient fuel to cross the barrier (Schaub et al. 2008).
Yet another factor that can influence stopover duration is weather. A number of studies have demonstrated that birds tend leave stopover sites when winds are favorable (tailwinds; Richardson 1990, Liechti and Bruderer 1998). However, precipitation can be a complicating factor; rain, for example, can saturate a bird’s plumage, increase wing loading, and increase rates of heat loss (Newton 2007a) so birds typically do not leave stopover sites during periods of precipitation. However, because early arrival times at breeding and wintering sites can be critically important, extended periods of harsh weather conditions (e.g., precipitation) can force birds to depart from a site even when weather conditions are not optimal, e.g., when there are head-winds (Erni et al. 2002, Jenni and Schaub 2003).

Figure 18. Example of the migration journey of a hypothetical bird, showing how distances flown and flight altitudes
can vary due to barriers like mountains and bodies of water as well as wind direction and velocity (with
variation indicated by the different-sized arrows; note that wind velocities are typically higher at higher altitudes).
(From: Åkesson and Hedenström 2007).
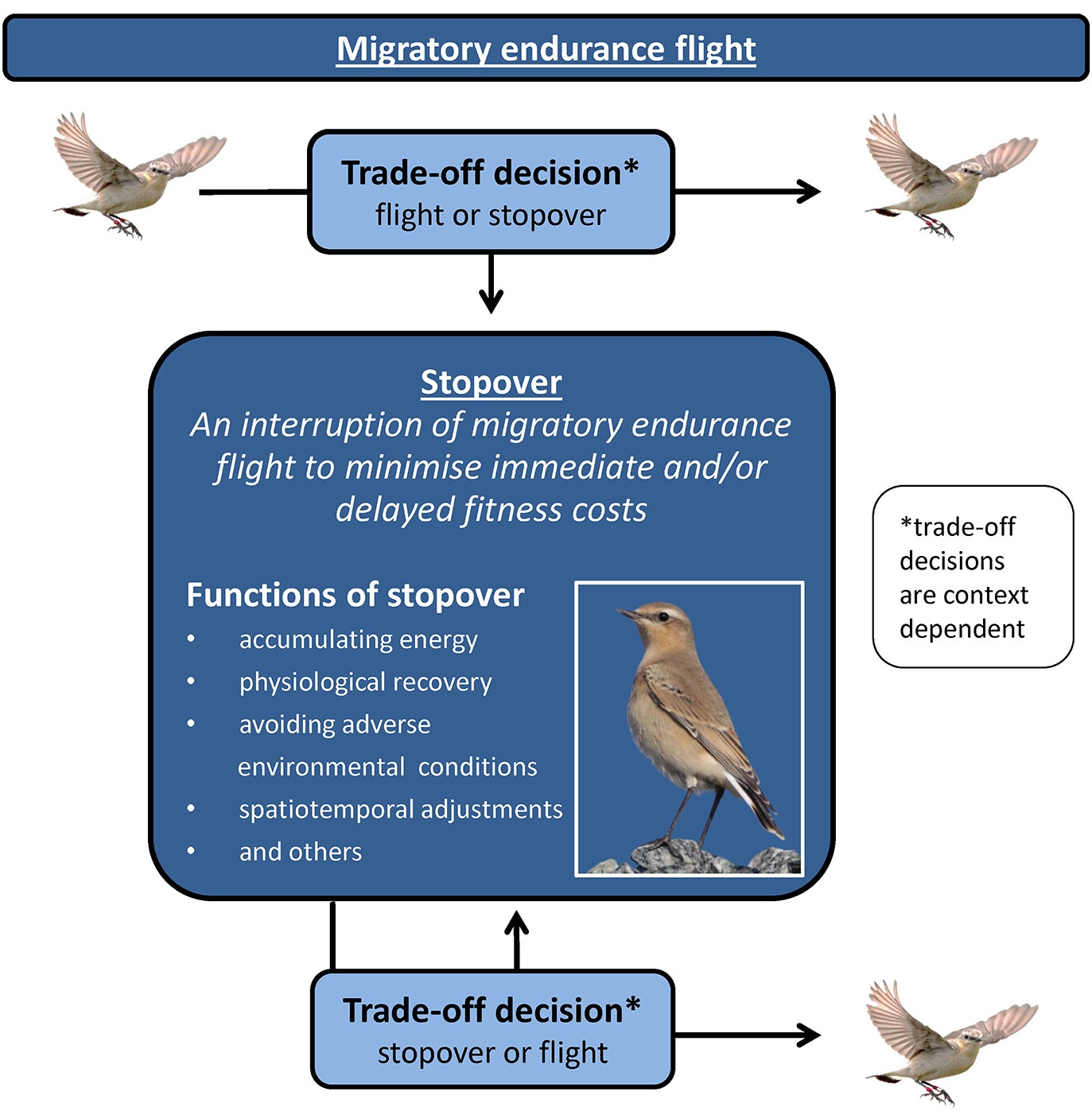
Diagram showing that migrants face a trade-off decision between continuing and interrupting migratory flights.
In the latter case, migrants use a stopover site. Some possible functions of stopovers are listed. During stopovers,
migrants face another trade-off between continuing stopover and resuming migration. Both trade-off decisions
should be considered in terms of ultimate fitness benefits (From: Schmaljohann et al. 2022).
Western Sandpiper migration
Understanding Stopover and Movement of Migratory Birds
 |
Decision to migrate and migration altitude varies with weather conditions. Doktor et al. (2010) used weather radar to study bird migration in western Europe and found that wind conditions influenced the altitudes at which birds flew. At four different locations with differing wind conditions, birds exhibited different migration strategies. At Trappes where there were favorable tail winds at higher altitudes (and unfavorable winds at lower altitudes), most birds quickly ascended to altitudes above 2 km. At Wideumont (280 km NE of Trappes), birds flew at altitudes below 2 km because winds were more favorable there than at higher altitudes. At De Bilt (north of Wideumont), few birds departed shortly after sunset due to a weak occlusion front (where a cold front overtakes a warm front) generated low clouds and precipitation a short distance to the south. After conditions became more favorable shortly after midnight (00.00), some birds took flight. Little bird activity was apparent at Den Helder. Such results clearly show how birds monitor weather conditions and adjust migration strategies accordingly. |
At stopover sites, birds in unfamiliar habitats that vary in suitability must forage to replenish fuel stores, likely in competition with resident birds as well as other migrants, while avoiding predators and, at times, seeking shelter during periods of inclement weather. Selection of optimal habitat is, therefore, very important. This can be particularly challenging, however, because birds, depending on their migratory pathways, may have to stopover in a variety of habitats, e.g., boreal forest, deciduous forest, deserts, savannahs, and so on.
Birds that migrate during the day can monitor habitats as they fly and choose as stopover sites those that appear suitable. However, many songbirds migrate at night and may land sometime during the night when visibility is limited. Landing sites for these night-migrating birds may be selected based on the visual cues that are available as well as acoustic cues (the calls of conspecifics and heterospecifics; Chernetsov 2006). After landing, migrants can, if necessary, move to more suitable habitats early the next morning. Selection of habitats for stopover may be condition dependent, with individuals needing to replenish fuel reserves more selective than those with adequate reserves that do not need to forage (Biebach 1990).
Migrants that gather reliable information about the unfamiliar habitats used during stopovers are more likely to forage efficiently and avoid predators. Some birds appear to combine personal information gathered at stopover sites with social information obtained by observing the behavior of other birds. Németh and Moore (2007) observed migrant songbirds along the Louisiana coast that had crossed the Gulf of Mexico and, by monitoring radio-tagged individuals, found that they were more likely to forage in flocks shortly after arrival (Figure 19). This suggests that migrants are using social information to learn about stopover sites and, perhaps, to increase their foraging efficiency.

Figure 19. Percentage of time spent in flocks by five radio-tracked Hooded Warblers (Wilsonia citrina) on the day
they first arrived at a stopover site and on their last day (From: Németh and Moore 2007).
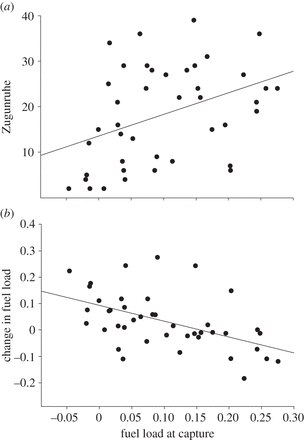
(a) Fuel load of Northern Wheatears at capture was positively correlated with Zugunruhe on the night following capture, and
(b) negatively correlated with the change in fuel load from capture to the third morning in captivity.
So, fat birds displayed
more Zugunruhe and accumulated less fuel than lean birds. Zugunruhe was
expressed as the number
of 15-min periods in a night
during which a bird showed at least five activity counts.
Fuel reserves at stopover correlated with nocturnal restlessness -- Early arrival at the breeding site positively affects the breeding success of migratory birds. During migration, birds spend most of their time at stopovers. Therefore, determining which factors shape stopover duration is essential to our understanding of avian migration. Because the main purpose of stopover is to accumulate fat as fuel for the next flight bout, fuel reserves at arrival and the accumulation of fuel are both expected to affect stopover departure decisions. Eikenaar and Schläfke (2013) determined whether fuel reserves and fuel accumulation predict a bird's motivation to depart, as quantified by nocturnal migratory restlessness (Zugunruhe), using Northern Wheatears (Oenanthe oenanthe) that were captured and temporarily contained at spring stopover. Fuel reserves at capture were found to be positively correlated with Zugunruhe, and negatively correlated with fuel accumulation. This indicates that fat birds were motivated to depart, whereas lean birds were set on staying and accumulating fuel. Moreover, the change in fuel reserves was positively correlated with the concurrent change in Zugunruhe, providing the first empirical evidence for a direct link between fuel accumulation and Zugunruhe during stopover. These results indicate that, together with innate rhythms and weather, the size and accumulation of fuel reserves shape stopover duration, and hence overall migration time.
The arrival times of migrating birds arrive at their breeding and wintering areas can be of critical importance. In spring in the northern hemisphere, birds arriving too early may face unfavorable weather (especially at higher latitudes), but those arriving too late may have reduced breeding success (Kokko 1999, Vergara et al. 2007). Many factors can influence the timing of migration, including genetic factors. For example, differences in the timing of fall migration by two species of redstarts, Common (Phoenicurus phoenicurus) and Black (P. ochruros) redstarts, were found to have a genetic basis (Berthold 1998). In a comparative analysis of 18 species, Berthold (1990) found that the onset of migratory activity in captive birds (migratory restlessness, or zugunruhe) maintained under controlled conditions was highly correlated with that of birds in the wild, suggesting that the timing of migration has a genetic, or innate, component. However, the extent of genetic control probably varies among species, with such control more likely in species, such as long-distance migrants, that breed in highly seasonal environments where environmental conditions are predictable within and across years (Ogonowski and Conway 2009) and when wintering and breeding areas are far apart and conditions in one location provide no evidence of conditions at the other location.
Among species where migratory behavior has a strong innate component, there must also be an internal clock or some internal mechanism for determining when to initiate migration. Such endogenous circannual clocks have been reported in more than 20 species of birds (Newton 2007b). The circannual clocks of birds have a period of about one year, but, for increased accuracy, are synchronized using environmental cues, such as daylength, light intensity, and seasonal rainfall patterns (Wikelski et al. 2008). Because it is highly predictable and consistent between years, most birds use changes in daylength to synchronize their internal clocks. Of course, variation in daylength varies with latitude and, near the equator, daylength changes little during the year. However, laboratory experiments indicate that even annual changes in daylength of one hour (equivalent to the change at 9° north or south of the equator) are sufficient for precisely synchronizing the circannual clocks of European Starlings (Sturnus vulgaris; Dawson 2007). Even more impressively, experiments revealed that even changes in daylength as little as 17 minutes produced behavioral and physiological changes in Spotted Antbirds (Hylophylax naevioides; Hau et al. 1998). Additional study is needed, but these results suggest than even birds near the equator may be able to use changes in daylength to synchronize circannual clocks.
Although the existence of circannual clocks has been well documented, how such clocks function remains unclear. One idea is that birds count days to derive an annual cycle (frequency de-multiplication hypothesis; Mrosovksy 1978, Gwinner 1986), but there is currently no experimental evidence to support this hypothesis. More recently, Wikelski et al. (2008) proposed an energy turnover hypothesis (ETH), where birds track the total amount of energy expended over a year and, by measuring or accounting for energy turnover (by some currently unknown mechanism), they can tell the time of year. This hypothesis remains to be tested. Regardless of how circannual clocks function, some birds clearly use such clocks to determine when to initiate migration.
For short-distance migrants, environmental factors have a greater influence on the timing of migration. When wintering and breeding areas are closer and where conditions in one location may provide some indication of expected conditions at the other location, environmental cues can be more useful. Environmental factors clearly influence the migratory behavior of irruptive species that do not migrate regularly. For example, decreasing food supplies triggers the migratory movements of Red Crossbills (Loxia curvirostra; Adkisson 1996) and other irruptive species of birds.
Regardless of the degree to which the timing of migration might be under genetic control, evidence suggests that birds can respond to a variety of cues during migration to better optimize arrival times. So, even for species where the time when migration begins is largely under genetic control, conditions en route can influence the duration of the migratory journey. Of course, some migrants must cross extensive areas of unsuitable habitat such as deserts or, for landbirds, extensive bodies of water and flights over those areas may often be or, in the case of large bodies of water for landbirds, must be crossed non-stop. After initiating such flights, birds sometimes, due to unfavorable winds or other factors (insufficient fat reserves), decide to return and reverse direction. For example, birds migrating from Europe to Africa and initiating flights across the Mediterranean Sea sometimes reverse course and return to land (reverse migration; Bruderer and Liechti 1998).
For long-distance migrants, migration typically consists of a series of flights, with those flights interspersed with time spent at stopover sites where energy reserves can replenished. Birds can potentially use environmental cues at stopover sites to determine how long to remain and what distance to fly before again stopping, and such decisions ultimately determine the overall speed of migration and the time of arrival at the breeding grounds. For example, Pink-footed Geese (Anser brachyrhynchus) appear to use plant phenology to determine speed of migration and arrival times in breeding areas, with these herbivores adjusting migration speed to more closely follow a green wave of plant growth (Duriez et al. 2009). Tøttrup et al. (2010) examined the timing of spring migration of 12 songbirds in Europe and found that local temperature best predicted arrival times in breeding areas. Interestingly, however, this was true only for individuals that were first to arrive in breeding areas, usually adult males closely followed by adult females. Thus, experienced birds monitored environmental conditions and timed their migration accordingly; inexperienced, first-time migrants relied entirely on endogenous, or innate, cues.
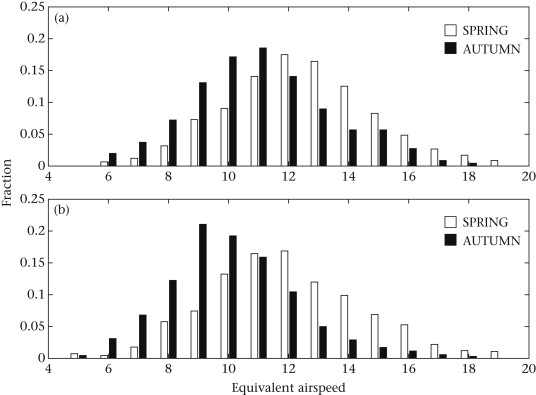
Distribution of equivalent airspeeds at (a) Lund (55°42′50″N) and (b) Abisko (68°21′13″N). The histograms show proportions of spring (open bars) and autumn (filled bars) equivalent airspeeds grouped by 1 m/sec categories (e.g. bars at 6 m/sec show the fraction of speeds in the interval 5.5–6.5 m/sec and so on).
Nocturnal migrants fly faster in the spring -- It has been suggested that time selection and precedence in arrival order are more important during spring than autumn migration. Migrating birds are expected to fly at faster airspeeds if they minimize duration rather than energy costs of migration, and they are furthermore expected to complete their journeys by final sprint flights if it is particularly important to arrive at the destination before competitors. Karlsson et al. (2011) tested these hypotheses by tracking-radar studies of nocturnal passerine migrants during several spring and autumn seasons at Lund (56°N) and Abisko (68°N) at the southern and northern ends of the Scandinavian Peninsula, respectively. The samples from these two sites represent migrants that are mostly rather far from (Lund) or close to (Abisko) their breeding destinations. Birds clearly flew at faster airspeeds in spring than in autumn at both study sites, with spring speeds exceeding autumn speeds by, on average, 16%, after taking effects of wind conditions and vertical flight speeds into account. This difference in speeds could not be explained by seasonal differences in body mass or wing morphology and thus supports the hypothesis of time-selected spring migration. There was also a significantly larger seasonal difference in airspeed at Abisko than at Lund, suggesting that the birds may have shown an inclination to sprint on their final spring flights to the breeding destinations, although this possible extra sprint effort was modest
Among many species of migratory birds, males tend to arrive in breeding areas before females, a phenomenon called protandry (Figure 20). Protogyny, where females arrive in breeding areas before males, has been reported only for species that exhibit sex-role reversal (e.g., phalaropes; Oring and Lank 1982, Reynolds et al. 1986). Among the hypotheses proposed to explain the evolution of protandry (and protogyny for sex-role reversed species) are the rank-advantage hypothesis and the mate-opportunity hypothesis. The rank-advantage hypothesis suggests that early arriving males benefit by acquiring higher-quality territories (Ketterson and Nolan 1976, Morbey and Ydenberg 2001). The mate opportunity hypothesis posits that early arriving males are more successful than later arriving males in acquiring mates; this would be particularly important for polygynous species and in populations with male-biased sex ratios where some males may not obtain mates (Morbey and Ydenberg 2001, Kokko et al. 2006). These hypotheses are, of course, not mutually exclusive because males (or females in sex-role reversed species) could benefit by both obtaining superior territories and being more likely to obtain mates.
If the outcome of male-male competition for the best territories influences male reproductive success and selects for protandry, then protandrous species might also be expected to exhibit sexual size dimorphism. This would be the case if larger males were more likely to outcompete smaller males. In addition, for species that breed at high latitudes where environmental conditions for early arriving migrants can be harsh, selection may also favor larger males better able to compete for access to limited food resources and, in addition, with smaller surface area-to-volume ratios and, therefore, better able to withstand cold conditions (Rubolini et al. 2004).
Of course, sexual size dimorphism might also be expected if mate acquisition is driving the evolution of protandry because larger males may outcompete smaller ones for access to females and females may prefer larger males. In addition, however, among songbirds, intense intersexual selection typically also favors male ornamentation, i.e., sexual dichromatism.
Rubolini et al. (2004) examined the relationship between the degree of protandry (the difference between male and female arrival times) and the degree of sexual dichromatism (differences between the plumage of males and females) and sexual size dimorphism for 21 species migratory songbirds. They found a strong, positive association between the degree of protandry and the degree of sexual dichromatism (Figure 21), but no relationship between the degree of protandry and sexual size dimorphism. In other words, the most protandrous species (where males tended to arrive at breeding grounds the earliest relative to females) were more sexually dichromatic, but not more sexually size dimorphic. Such results are consistent with the predictions of the mate opportunity hypothesis and suggest that increased success at acquiring mates may be a major driving force in the evolution of protandry. In further support of the mate opportunity hypothesis, Kokko et al. (2006) generated models in an attempt to better understand the selective pressures favoring protandry and also found that the mate-opportunity hypothesis better explained the evolution of protandry than the rank-advantage hypothesis. Their models suggest that competition for territories alone is not sufficient to favor the evolution of protandry; early arrival must also improve mating opportunities.
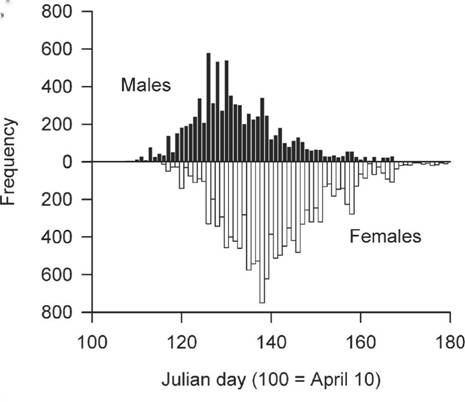
Figure 20. Protandry in Common Redstarts (Phoenicurus phoenicurus), with males beginning to arrive in
breeding areas several days before the first females arrive (From: Coppack and Pulido 2009).
Figure 21. Relationship between standardized difference in migration dates between females and males
(greater values indicate that males are migrating earlier than females) and degree of sexual dichromatism
(scored on a 0–24 scale) for 21 species of long-distance migratory songbirds (From: Rubolini et al. 2004).
Diurnal vs. nocturnal migration
Many species of birds migrate at night, but some migrate during and day and still others are flexible and can migrate at any time of day. Most waterfowl, including ducks and geese, are flexible and migrate at various times of day. However, most long-distance migratory songbirds and shorebirds migrate only at night, and some songbirds, including finches, swallows, and corvids, as well as pigeons and doves migrate only during the day (Alerstam 1990).
An important advantage of nocturnal migration is that, for birds that forage during the day, more time is available for foraging. Nocturnal migrants can initiate migration after a day spent foraging and storing energy, whereas diurnal migrants must balance flight time and foraging during the day and spend the night roosting and sleeping. As a result, nocturnal migrants can spend more time flying, resulting in faster migration (Alerstam 2009). The potential importance of maximizing foraging time is perhaps best illustrated by shorebirds. Lank (1989) found that shorebirds typically initiate migratory flights at dusk, after light levels made foraging difficult, but also noted that shorebirds sometimes initiated migratory flights during the day if foraging at a particular location is prevented (e.g., due to rising tides).
Beyond increased foraging and flight time, nocturnal migration may also be advantageous because flying conditions are typically better, with less atmospheric turbulence, less wind, and reduced evaporative water loss (because temperatures are cooler). Flying at night may also take less energy because, with cooler temperatures and higher humidity increasing air density, generating lift is easier (Kerlinger and Moore 1989, Alerstam 2009). In addition, with few or no aerial predators, predation risk is lower for birds migrating at night (although, in some areas, birds risk predation by bats; see below). Finally, many birds use navigational cues that are only available at dusk or at night (e.g., stars).
This animation created by Cornell University researchers illustrates the use of a network of surveillance weather radar
to record nocturnal migrating birds, bats, and insects in the continental U.S. from sunset to sunrise on 1 October 2008. The
blocky green, yellow, and red patterns, especially visible on the east coast, represent precipitation, but, within an hour
after sunset, radar picks up biological activity, as seen in the widening blue and green circles spreading from the east
across the country. Birds take off, fly past, and get sampled by the radar beam. Note, the black areas on the map do not
represent places without birds, necessarily, but rather places where radar does not sample
Bats preying on migrating birds. Although bats preying on birds has rarely been documented, examination of 14,000 fecal pellets revealed that greater noctule bats (Nyctalus lasiopterus) capture and eat large numbers of migrating songbirds in Spain. Ibáñez et al. (2001) reported that these bats likely capture and eat birds in flight, just as aerial-hawking bats normally do with insects. Bats can approach and surprise birds without being detected because their echolocation calls are well above the frequencies songbirds can detect. Greater noctule bats are one of the largest aerial-hawking bats in the world, with a mean mass of 48 grams and typical wingspan of 45 cm, so can easily overpower small songbirds flying at night.
|
Given the many advantages of nocturnal migration, why do some birds migrate during the day? In contrast to the flapping flight of nocturnal migrants, some species, such as hawks, vultures, and storks, soar and glide during migration. Soaring requires less energy than flapping flight, but requires rising currents of air that are generating during the day, either by unequal heating of the earth’s surface (thermals) or by wind directed upward by mountain ranges or other obstacles (obstruction lift). Of course, birds that migrate during the day can also forage, using a fly-and-forage strategy. For example, Hobbies (Falco subbuteo) monitored by satellite tracking were found to occasionally fly at lower speeds, especially during the afternoon, with reduced speed suggesting a temporary switch to focus on hunting (Strandberg et al. 2009). Aerial insectivores, such as swallows, may also use a fly-and-forage strategy, occasionally spending time foraging during their migration flights. Depending on the migration route, birds may be limited in their use of the fly-and-forage strategy. For example, Barn Swallows migrating from breeding areas in Europe to wintering areas in Africa, must, like long-distance nocturnal migrants, build up fat stores before making long flight across the Mediterranean Sea and the Sahara Desert (Rubolini et al. 2002).
For nocturnal migrants, internal clocks and sunset cues determine when migratory flights begin each evening. For example, wild-caught migratory Common Redstarts (Phoenicurus phoenicurus) had captive for three days without any photoperiodic cues and a constant supply of food still exhibited clear patterns of migratory activity, with the onset of migration each night coinciding with the time of local sunset (Coppack et al. 2008). If redstarts and other species of birds initiated nightly migratory flights based on factors such as habitat quality, physical condition, or experience, then they would not exhibit such consistent temporal patterns, regardless of age or point of origin (Coppack et al. 2008). In contrast to the onset of migratory flights, birds exhibit much more variation in when such flights end. Flights can end before, at, or after sunrise and that timing likely depends on a number of factors, including physical condition, weather and flight conditions, and the need to land in suitable habitat.
Most of what is known about the migratory behavior of birds is based on studies of birds that breed in the Northern Hemisphere and migrate south during the non-breeding season. Some birds that breed in the southern hemisphere also migrate (Figure 22), but much less is known about their migratory behavior. In South America, the annual movement of birds from breeding areas in temperate areas to non-breeding areas at lower latitudes is called austral migration. There are approximately 230 species of austral migrants (compared to about 340 species of Nearctic-Neotropical migrants; Rappole 1995), with most (about 120 species) being flycatchers (Tyrannidae). Among Nearctic-Neotropical migrants, most are warblers (about 210 species, Parulinae; Chesser 2005). There are fewer austral migrants because southern South America is much smaller in area than northern North America (about 5 times smaller; Chesser 1994).

Fig. 22. Breeding and wintering ranges of Slaty Elaenias (Elaenia strepera) in South America. The breeding range and probable winter range
are about 3000 km apart. Dots indicate locations where individuals were observed (From: Marantz and Remsen 1991).
Compared to Nearctic-Neotropical migrants, austral migrants tend to migrate shorter distances and, in many cases, breeding and wintering ranges overlap (Fig. 23). Only about 7% of austral migrants have completely separate breeding and wintering areas (Stotz et al. 1996). The average distance migrated by Austral migrants equals 9.2 degrees in latitude, whereas, for Nearctic-Neotropical migrants, the average distance migrated equals 22.5 degrees in latitude (Chesser 2005). Because most of the land area in South America is located near the equator than in North America, birds need not migrate as far. Because of the distribution of land area in South American, there is far more wintering area available than breeding area. In North America, in contrast, there is far more breeding area available than wintering area. In addition to the shorter migration distances, Austral migrants, in contrast to many Nearctic-Neotropical migrants that must cross the Gulf of Mexico, face no geographic barriers. Interestingly, although many species of birds that breed in temperate areas of North America winter in temperate areas in South America (e.g., several species of shorebirds), there are no birds that breed in the south-temperate zone that winter in the north-temperate zone (Chesser 1994, Jahn et al. 2004). Why this is the case is unclear (Jahn et al. 2004).
Ecologically, most austral migrants breed in open habitats like grasslands, whereas most Nearctic-Neotropical migrants breed in forested habitats (Jahn et al. 2004). In both cases, however, migrants tend to be habitat generalists during the non-breeding season (Jahn et al. 2004).
Fig. 23. The breeding and winter ranges of Tropical Kingbirds in South America overlap. The gray area mostly north
of 18°S latitude represents the area where they are found year-round. The dashed line within the gray area indicates the Amazon Basin.
The white area indicates where some Tropical Kingbirds breed during the austral summer (From: Jahn et al. 2010).
Afro-Palaearctic landbird migration. With >100 species and more than two billion individuals travelling
annually, the Afro-Palaearctic bird migration system is the largest
landbird migration network in the world. Songbirds and near-passerine birds make up >80% of all Afro-Palaearctic migrants. Generally, long-distance migrants travel between European breeding and sub-Saharan non-breeding grounds via several broad-scale migration corridors and flyways that are channelled
through specific geographical locations. These flyways have been formed through the combined effects of geographical bottlenecks and the post-glacial colonization from glacial refugia in the Iberian and Balkan peninsulas.
Briedis et al. (2020) compiled an individual-based dataset for 23 passerine and
near-passerine species of 55 European breeding populations, with a total of 564
individuals tracked during migration between Europe and sub-Saharan Africa. Analysis revealed that the migration routes of Afro-Palaearctic migrants consist of two broadly defined flyways, which converge between about 10 and 20° E in central Europe. The birds mainly follow a longitudinally parallel migration pattern, and northern breeders surpass southern breeders at the sub-Saharan non-breeding sites (i.e., they perform leapfrog migration).
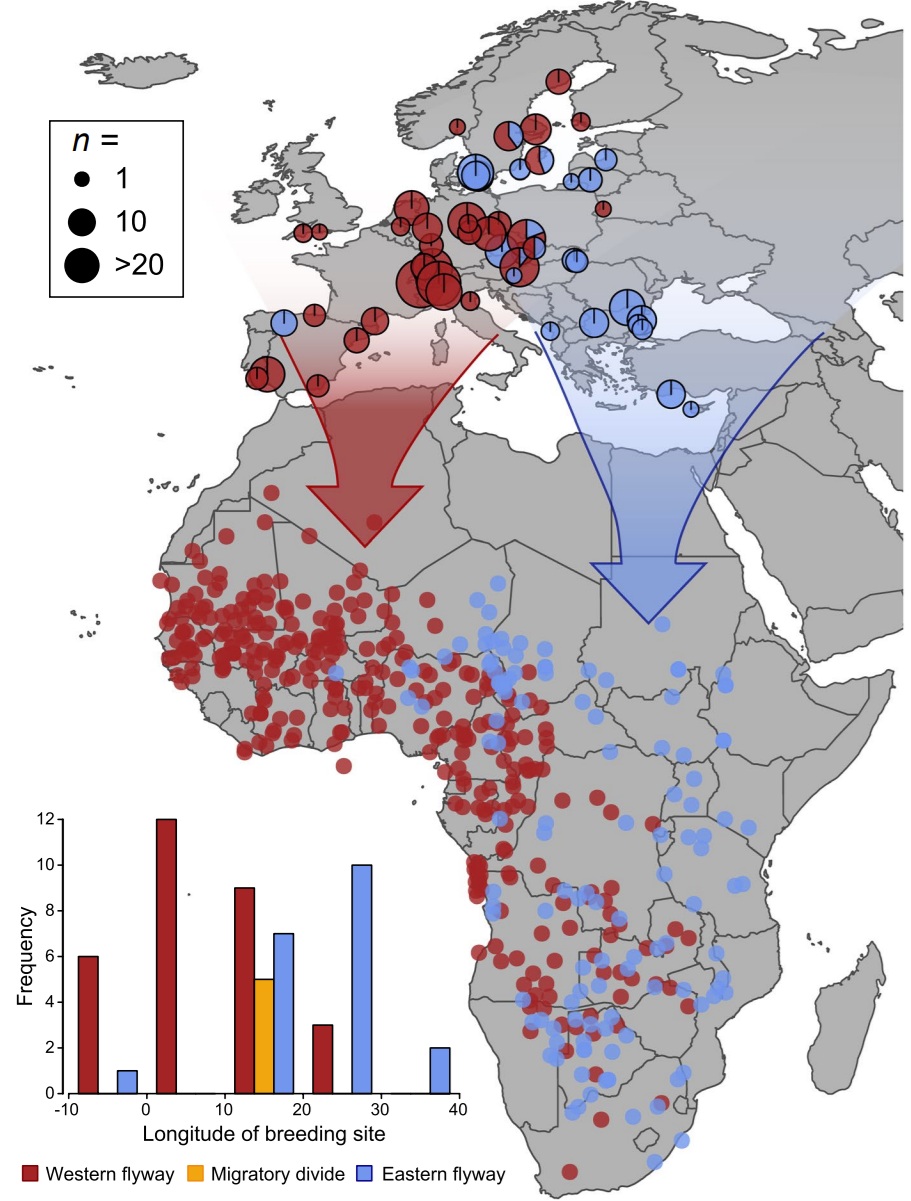
Flyway use of Afro-Palaearctic long-distance migrants (red = Western flyway; blue = Eastern flyway). Pie charts
mark the breeding site locations [diameter indicates sample size (n)] and show the proportions of
individuals that use
either of the flyways within each population. Individual wintering sites are marked with dots. The inset shows the frequency
distribution of flyway use according to breeding site longitude (values are binned into 10° longitudinal bands). The
orange bar indicates populations that use both flyways (i.e., populations with a migratory divide).
Briedis et al. also found that the longitudes of individual breeding and non-breeding sites were related in a strongly positive manner, whereas the latitudes of breeding and non-breeding sites were related negatively. In autumn, migration began before vegetation senescence, and the timing of migration was 5–7 days earlier along the Western flyway than the Eastern flyway. In spring, the time of arrival at breeding sites was about 1.5 days later for each degree northwards and 6–7 days later along the Eastern than the Western flyway, reflecting the later spring green-up at higher latitudes and more eastern longitudes. In general, migration by Afro-Palaearctic landbirds follows a longitudinally parallel leapfrog migration pattern, with migrants tracking vegetation green-up in spring, but departing before vegetation senescence in autumn.
Songbird migration between Eastern Europe and Southern Asia. The arid belt of Central Asia constitutes an ecological barrier that migratory landbirds have to negotiate twice a year (Figure below). The severity of this barrier differs between spring and autumn: in spring, the conditions are relatively benign, whereas during autumn passage (August–October) temperatures are high, humidity is low, and green vegetation is sparse and restricted to oases and agricultural areas. Some species that migrate from Siberia to Africa circumvent this barrier by taking a detour around the northern coast of the Caspian Sea. However, some songbirds that migrate between eastern Europe and southern Asia may also take a detour along the northrn edge of the desert. Chernetsov et al. (2025), using trapping data from the northern fringe of the barrier and from earlier studies conducted further west and east, showed that some songbirds that migrate from European breeding grounds towards south and southeastern Asian wintering areas make a direct crossing of the arid belt (e.g., Bluethroats, Red-breasted Flycatchers, Blyth's Red Warblers, and Common Rosefinches), whereas others take a detour and circumvent Central Asian deserts (e.g., Greenish Warblers and Paddyfield Warblers). Ecological differences between the species of migrants cannot explain the observed pattern. Those species that make a detour may be ones that colonized Europe from their ancestral breeding areas east of the Ural Mountains.
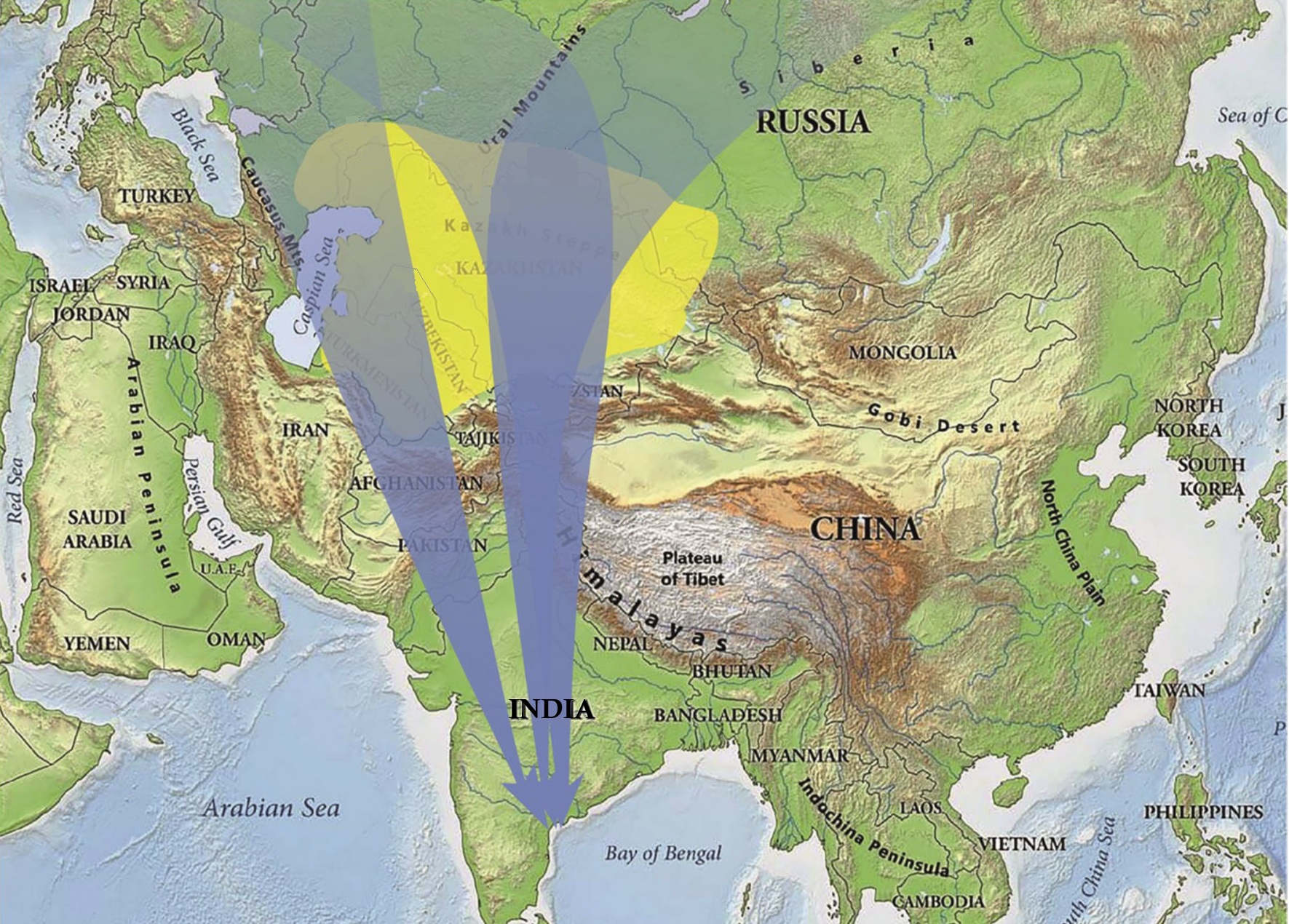
Schematic drawing of Eurasia showing the suggested migratory routes of songbird migrants from Eastern
Europe and West Siberia to Indian winter quarters. The approximate extent of the arid belt is highlighted in yellow.
East Asian Migratory Flyway
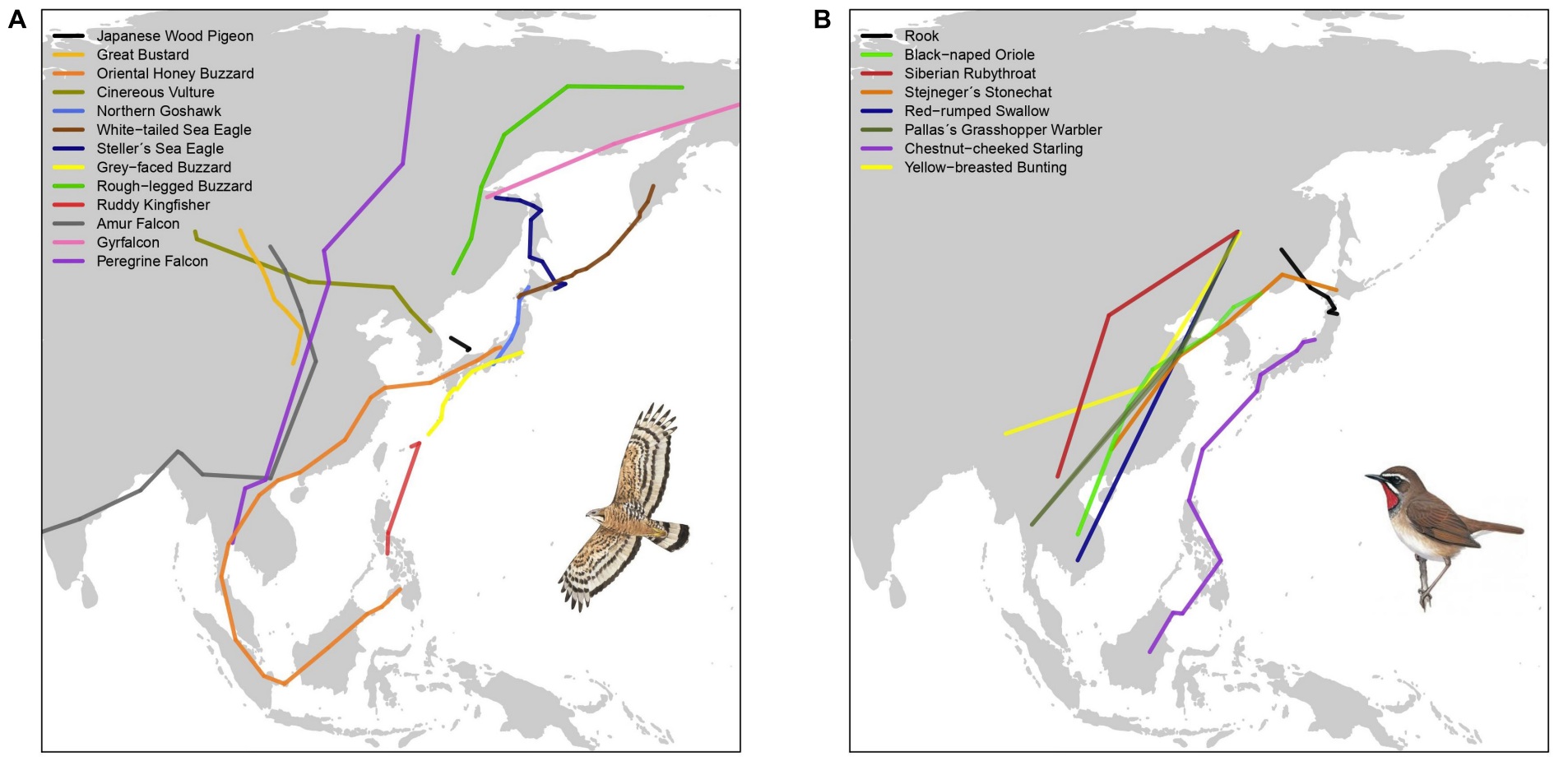
Autumn migration routes of landbirds in the East Asian Flyway, including one individual track for each species where data
are available (n = 21). (A) Non-passerines (illustration of Oriental Honey Buzzard), (B) Passerines (illustration of Siberian Rubythroat).
With nearly 400 migratory landbird species, the East Asian Flyway is the most diverse of the world’s flyways. This diversity is a consequence of the varied ecological niches provided by biomes ranging from broadleaf forests to Arctic tundra and accentuated by complex biogeographic processes. The distribution and migration ecology of East Asian landbirds is still inadequately known, but a recent increase in the number of studies tracking the migration of raptors, cuckoos, kingfishers and passerines has increased our knowledge about the stopover and wintering ecology of many species, and the migratory routes that link northeast Eurasia and the Asian tropics. The East Asian Flyway also supports the highest number of threatened species among flyways. Strong declines in population have been detected in buntings (Emberizidae) and other long-distance migrants. Although the conservation of migratory landbirds in this region has largely focused on unsustainable hunting, there are other threats, such as habitat loss and increased agro-chemical use driven directly by land cover change and climate-related processes. Important knowledge gaps to be addressed include (1) threats affecting species in different parts of their annual cycle, (2) range-wide population trends, (3) ecological requirements and habitat use during the non-breeding season, and (4) the conservation status of critical wintering sites (including understudied farming landscapes, such as rice fields) and migration bottlenecks along the flyway (From: Yong et al. 2021).
Migration routes of long-distance migrating seabirds. Approximately 76% of seabirds are classified as ‘full-migrants’, meaning a substantial proportion of the population makes regular or seasonal cyclical movements beyond the breeding range, with predictable timing and destinations. Migratory seabirds can be further classified by their foraging habitat: coastal or pelagic. Many coastal migratory seabird species travel migration routes that are entirely coastal or terrestrial, that are already encompassed within the existing flyways. However, 58.6% of seabirds are classified as pelagic, meaning they primarily use deep marine waters (typically >200 m in depth), or neritic, i.e., continental shelf waters. Key oceanic migratory routes have been identified for some species, such as the figure-8 migration paths in the Pacific and Atlantic oceans, or the circumnavigations of the Southern Ocean, but these routes are currently outside of any existing flyways classifications. Morten et al. (2025) used a comprehensive global tracking dataset that included the migratory routes of 48 pelagic and long-distance migrating seabird species across the Atlantic, Indian, Pacific and Southern Oceans to identify individuals that followed similar routes, independent of species or timings of migration. Analysis revealed six marine flyways in the four ocean basins: Atlantic Ocean Flyway, North Indian Ocean Flyway, East Indian Ocean Flyway, West Pacific Ocean Flyway, Pacific Ocean Flyway, and Southern Ocean Flyway.
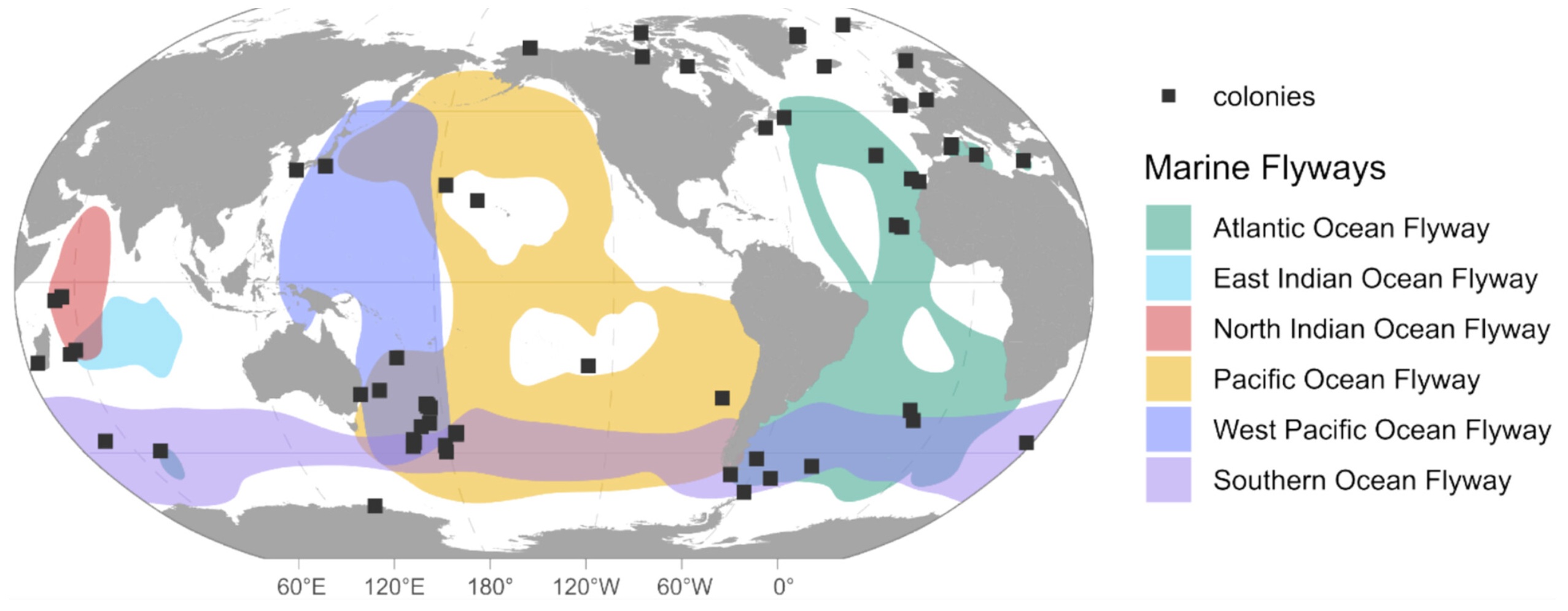
Six marine flyways identified across four ocean basins identified from analysis of tracking data for 48 pelagic seabird species breeding at the 64 colonies indicated by the black squares.
The six marine flyways cover extensive areas of the global ocean, and although very broad, illustrate the vast movements and connectivity performed by seabird populations and species during their annual cycle, mirroring the terrestrial and coastal flyways. The marine flyways are used bidirectionally and throughout the year, depending on breeding sites and phenologies. Seabird migrations are strongly affected by wind patterns and surface ocean currents. Individuals may delay departure for migration in response to wind conditions and adjust routes to minimize energetic costs by using tail winds to assist flight and avoid head winds. The six marine flyways follow the major prevailing winds. For example, the Atlantic Ocean Flyway and Pacific Ocean Flyway figure-8 routes correspond with the circular patterns of ocean circulation in each hemisphere, and the west to east movements in the Southern Ocean Flyway also correspond with ocean circulation patterns. The six marine flyways illustrate the migration routes shared by pelagic seabirds at ocean-basin scales and highlight the need to account for ecological connectivity in seabird conservation.
Bird migration and climate change
For birds that breed at mid- to high latitudes, the time of arrival on their breeding areas has been selected over time so they largely miss the adverse weather conditions of late winter and early spring and arrive when food availability is increasing. Over the past several decades, however, global temperatures have increased due to anthropogenic climate change, with corresponding changes in the timing of seasonal climate conditions. These changes in temperature have not been uniform across the globe; temperatures in the Northern Hemisphere, particularly at higher latitudes, have increased more than those in the Southern Hemisphere, and temperatures in Europe and northern Asia have increased more than those in most of North America (excluding parts of Alaska; Figure 24).
Figure 24. Increases in temperatures during the period from 2000 to 2009 compared to average temperatures recorded between 1951 and 1980.
The most extreme warming (shown in red) was in the Arctic. Few areas had cooler temperatures (shown in blue). Gray areas over parts of the
Southern Ocean are places where temperatures were not recorded. Note that temperatures have increased more in Europe and Asia
than in North America. (Source: http://en.wikipedia.org/wiki/File:GISS_temperature_2000-09_lrg.png).
Not surprisingly, increasing temperatures have altered the timing or phenology of biological processes in many areas, but particularly at higher latitudes in the Northern Hemisphere. For example, in the Arctic, where annual average temperatures have increased at almost twice the rate of the rest of the world (Callaghan et al. 2005; Figure above), warmer winters and earlier springs have advanced the date of peak abundance of arthropods by about 7 days (Tulp and Schekkerman 2008). Climate change has also altered the phenology of migration for many species of birds. For example, Végvári et al. (2010) determined first-arrival dates (the day on which the first individuals of a migratory species is observed in the spring) of migrants in eastern Hungary (47°N latitude) and found that, during the period from 1969 to 2007, the time of arrival was significantly earlier for 45 of 177 species (25.4%; Figure 25). First-arrival dates of short-distance migrants (species wintering north of the Sahara Desert) were advanced more than those of long-distance migrants (species wintering south of the Sahara in tropical Africa) (Figure 25a below). The shorter the migration distance, the greater the tendency for earlier arrival dates (Figure 25b below). Similarly, Murphy-Klassen et al. (2005) examined a 63-year dataset (1939 to 2001) of first spring sightings for 96 species of birds in Manitoba (50° N latitude) and found that 25 species (26%) had significant trends for increasingly early arrival. As also found in Hungary, more short-distance migrants (33.2% of species) exhibited significant tendencies for earlier arrival dates than long-distance migrants (18.8% of species).
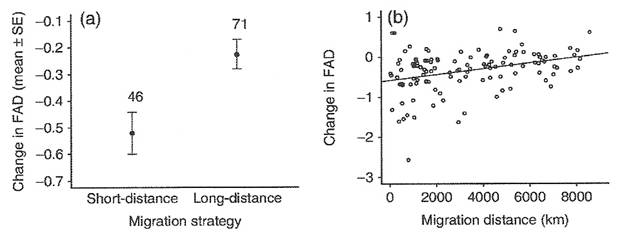
Figure 25. Annual change (days per year) in the date when the first individuals of migratory birds were observed in eastern Hungary relative to
(a) migration strategy and (b) migration distance. Numbers above error bars in (a) indicate the number of species (From: Végvári et al. 2010).
Although studies have revealed that many species of migratory birds are now arriving on their breeding grounds earlier than in the past, this trend is by no means universal because many other species have exhibited no change in the timing of migration. A number of factors might help explain differences among migratory species in their response to climate change, including habitat, feeding ecology, breeding behavior, migratory strategy (e.g., distance between wintering and breeding areas), and structural traits such as body size (Lehikoinen and Sparks 2010). In a comparative study that included data from 117 species of birds, Végvári et al. (2010) found that advancement of first-arrival dates was greater in migratory species with more generalized diets, shorter migration distances, more broods per year, and less extensive prebreeding molt. As also noted in a number of previous studies (e.g., Murphy-Klassen et al. 2005), Végvári et al. (2010) found that one of the most important factors in predicting the response of a species to climate change was migration distance, with short-distance migrants advancing first-arrival dates more than long-distance migrants. A likely explanation for this difference is that the timing of migration for long-distance migrants is under stronger endogenous control (e.g., responding to changes in daylength) whereas short-distance migrants respond more to variation in climate. On their distant wintering areas, long-distance migrants cannot predict weather conditions on their breeding grounds. For short-distance migrants, weather conditions in wintering areas are more likely to be related to conditions in breeding areas, allowing them adjust the timing of migration accordingly. Diet, number of broods per year, and molting strategies also appear to influence migration strategies (Végvári et al. 2010). Birds with more general diets are likely better able to find sufficient food during migration and after arrival in breeding areas than those with specialized diets and, therefore, are better able to respond to climate change by advancing the timing of migration. Because changes in global temperatures have not been uniform and specific weather conditions vary between and within years, generalized diets would be beneficial for birds migrating earlier because the availability of certain types of food (e.g., arthropods, Tulp and Schekkerman 2008) may vary geographically (e.g., along migration routes) and temporally (e.g. between years). In contrast, birds with specialized diets would be less likely to survive if variable weather conditions reduced availability of their required foods. The time of arrival on breeding grounds for multibrooded species has tended to advance more than that of species that typically produce fewer broods (Møller et al. 2008, Végvári et al. 2010). Species that can potentially raise multiple broods during a breeding season are under stronger selection for earlier arrival (providing more time for more broods), accelerating their response to a warming environment (Végvári et al. 2010). First-arrival dates of species with no prebreeding molt have advanced more that those species with a prebreeding molt and those that molt flight feathers in wintering areas (Végvári et al. 2010). Birds with a prebreeding molt are unable to initiate migration before completing molt because the energetic costs of replacing feathers while migrating would be too high. The need to complete molt before migrating, therefore, means that the timing of migration for these species is less flexible than for species with no prebreeding molt. Previous studies have revealed conflicting results concerning the possible effect of habitat on the responses of birds to climate change. Végvári et al. (2010) found no effect of habitat on first-arrival dates. However, Butler (2003) found that the first-arrival dates of grassland birds were more advanced than those of birds that breed in other habitats. Most grassland birds are omnivores and feed on seeds when other types of prey (e.g., insects) are less available. With warming temperatures, snow cover may melt sooner, exposing underlying vegetation and seeds and providing early arriving grassland birds with a source of food (Butler 2003). By contrast, arrival times of species that feed primarily on arthropods (e.g., many forest-dwelling birds) are more constrained by the time of emergence of their food source. Although global warming has clearly affected the timing of spring migration of many species of birds, studies in both Europe and North America have generally revealed no consistent trends in the timing of fall migration. Sparks et al. (2007) examined departure dates of 23 species and a trend for slightly later departure, but few species exhibited significant changes in departure dates. Similarly, Van Buskirk et al. (2009) reported conflicting results, with some species tending to depart later and some earlier; no variable (e.g., migration distance or number of broods) was significantly associated with departure date. The effect of global warming on the timing of fall migration by birds is less clear-cut because, in contrast to the clear relationship between fitness and early spring arrival on the breeding grounds (Møller 1994, Nystrom 1997), fitness considerations in the fall might favor earlier departure, later departure, or no change in departure time (Mills 2005). For example, if global warming allows earlier breeding (and the earlier completion of breeding), then species where individuals compete for winter territories might be expected to leave breeding areas earlier, e.g., Yellow Warblers (Dendroica petechia; Mills 2005). However, global warming may lengthen the breeding season, permitting, for example, more broods, additional time between broods, or more time to complete post-breeding molt before initiating migration. In each of these cases, fall migration might be delayed, resulting in later departure dates. Finally, for some species, such as long-distance migrants, the timing of migration may be endogenously fixed and, as a result, global warming will have no impact on the timing of fall migration (Mills 2005). Although responses vary, global warming has clearly affected the timing of spring migration for many species of birds. Because selection has favored individuals that time their arrival in breeding areas so that the time of peak food demand (feeding young) matches the period of peak food availability, one potential consequence of changes in the timing of migration is a mismatch between the timing of food demand and availability (Figure 26). For example, as noted previously, warmer winters and earlier springs have advanced the date of peak abundance of arthropods in some areas of the Arctic tundra by about 7 days (Tulp and Schekkerman 2008). If arrival dates of migrant birds are not similarly advanced, then the time of peak food demand may no longer match the time of peak availability and such a mismatch could have a negative impact on breeding success. Such a mismatch has apparently contributed to a decline in populations of long-distance migrants that breed in forested habitats in Europe (Both et al. 2010) and, more generally, migrant bird populations in both the Nearctic and Palearctic (Jones and Cresswell 2010). In general, species of birds that breed in habitats characterized by short bursts of food availability (e.g., temperate forests and tundra) and where the timing of migration is controlled by endogenous factors (long-distance migrants) are most likely to be negatively impacted by such mismatches (Both 2010, Both et al. 2010). Short-distances migrants are better able to adjust the timing of migration and avoid or minimize mismatches between food demand and availability. Similarly, birds that breed in habitats characterized by longer peaks in food availability (e.g., marshes and coniferous forests) are less likely to be impacted by such mismatches (Both et al. 2010). Available evidence indicates that a mismatching of food demand and availability is having a negative impact on populations of some species of migratory birds, but relationships between global warming, food availability, and timing of bird migration are complex. For example, species of birds breeding in the same habitat may experience different mismatches or be able to avoid such mismatches due to subtle differences in food habits (Jones and Cresswell 2010). In addition, climate change may cause shifts in or expansion of breeding and wintering ranges, affecting the degree to which food demand and availability might or might not mismatch. Clearly, additional study is needed to better understand how global warming is impacting the behavior, breeding success, and population status of migratory birds. Although increasing global temperatures have clearly altered the migratory behavior of many species of birds, the potential future impacts of global warming on birds are likely to be even more pronounced. If, as predicted by some, global mean temperatures increase by up to 8 degrees C by the year 2100 (Figure 27), the resulting changes in climate and sea levels will undoubtedly have tragic consequences for many species of birds. Six decades of North American bird banding records reveal plasticity in migration phenology -- The timing of avian migration has evolved to exploit critical seasonal resources, yet plasticity within phenological responses may allow adjustments to interannual resource phenology. The diversity of migratory species and changes in underlying resources in response to climate change make it challenging to generalize these relationships. Horton et al. (2023) used bird banding records during spring and fall migration from across North America to examine macroscale phenological responses to interannual fluctuations in temperature and long-term annual trends in phenology. In total, 19 species of North American wood warblers (family Parulidae) were examined, summarizing migration timing from 2,826,588 banded birds from 1961 to 2018 across 46 sites during spring and 124 sites during fall. During spring, warmer spring temperatures at banding locations translated to earlier median passage dates for 16 of 19 species, with an average 0.65-day advancement in median passage for every 1°C increase in temperature, ranging from 0.25 to 1.26 days per °C. During the fall, relationships were considerably weaker, with only 3 of 19 species showing a relationship with temperature. In those three cases, later departure dates were associated with warmer fall periods. Projecting these trends forward under climate scenarios of temperature change, the authors forecast continued spring advancements under shared socioeconomic pathways from 2041–2060 and 2081–2100 and more muted and variable shifts for fall. These results demonstrate the capacity of long-distance migrants to respond to interannual fluctuations in temperatures, at least during the spring, and showcase the potential of North American bird banding data for understanding phenological trends across a wide diversity of avian species.

Figure 26. Example of a mismatch caused by global warming. Before global warming (above), environmental cues (dashed line) that triggered the onset
of egg-laying (daylength) resulted in nestlings being present when food availability (caterpillars) was highest. With global warming (below), food
availability now peaks prior to peak food demand (nestlings present) because the cues that trigger egg-laying (daylength) are no longer synchronized
with the cues that trigger hatching and development of caterpillars (temperature) (From: Durant et al. 2007). 
Figure 27. Predicted distribution of temperature change due to global warming from
the Hadley Centre HadCM3 climate model
(Source: http://www.globalwarmingart.com/wiki/File:Global_Warming_Predictions_Map_jpg).
Able, K. P., and J. R. Belthoff. 1998. Rapid ‘evolution’ of migratory behaviour in the introduced House Finch of eastern North America. Proceedings of the Royal Society of London B 265: 2063-2071.
Adkisson, C. S. 1996. Red Crossbill (Loxia curvirostra). The Birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology, Ithaca, NY. Retrieved from the Birds of North America Online <http://bna.birds.cornell.edu/bna/species/256 >
Åkesson, S., and A. Hedenström. 2007. How migrants get there: migratory performance and orientation. BioScience 57: 123-133.
Alerstam, T. 1990. Bird migration. Cambridge University Press, Cambridge.
Alerstam, T. 2001. Detours in bird migration. Journal of Theoretical Biology 209: 319-331.
Alerstam, T. 2009. Flight by night or day? Optimal daily timing of bird migration. Journal of Theoretical Biology 258: 530-536.
Alerstam, T., A. Hedenström, and S. Åkesson. 2003. Long-distance migration: evolution and determinants. Oikos 103: 247-260.
Alves, J. A., T. G. Gunnarsson, P. M. Potts, G. Gelinaud, W. J. Sutherland, and J. A. Gill. Overtaking on migration: does longer distance migration always incur a penalty? Oikos 8: 464-470.
Barcante et al. 2017. Altitudinal migration by birds: a review of the literature and a comprehensive list of species. Journal of Field Ornithology 88: 321-335,
Barlein, F., D. R. Norris, R. Nagel, M. Bulte, C. C. Voigt, J. W. Fox, D. J. T. Hussell, and H. Schmaljohann. 2012. Cross-hemisphere migration of a 25 g songbird. Biology Letters 8: 505–507.
Bell, C. P. 1997. Leap-frog migration in the Fox Sparrow: minimizing the cost of spring migration. Condor 99: 470-477.
Belthoff, J. R., and S. A. Gauthreaux, Jr. 1991. Partial migration and differential winter distribution of House Finches in the eastern United States. Condor 93: 374-382.
Berthold, P. 1990. Wegzugbeginn und einsetzen der zugunruhe bie 19 vogelpopulationen – eine vergleichende untersuchung. In: Proceedings of the International Centennial Meeting of the DO-G, Current topics in avian biology (R. van den Elzen, K.-L. Schuchmann, and K. Schmidt-Koenig, eds.), pp. 217-222. Bonn, Germany.
Berthold, P. 1998. Bird migration: genetic programs with high adaptability. Zoology 101: 235-245.
Berthold, P. 1999. A comprehensive theory for the evolution, control and adaptability of migration. Ostrich 70: 1-11.
Berthold, P. 2001. Bird migration: a general survey. Oxford University Press, Oxford, UK.
Biebach, H. 1985. Sahara stopover in migratory flycatchers: fat and food affect the time program . Experientia 41: 695 - 697 .
Biebach, H. 1990. Strategies of trans-Sahara migrants. In: Bird migration: physiology and ecophysiology (E. Gwinner, ed.), pp. 352-367. Springer-Verlag, Berlin, Germany.
Both, C. 2010. Food availability, mistiming, and climatic change. In: Effects of climate change on birds (A. P. Møller, W. Fiedler, and P. Berthold, eds.), pp. 129-147. Oxford University Press, New York, NY.
Both, C., C. A. M. Van Turnhout, R. G. Bijlsma, H. Siepel, A. J. Van Strien, and R. P. B. Foppen. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of the Royal Society B 277: 1259-1266.
Boulet, M., and D. R. Norris. 2006. The past and present of migratory connectivity. Ornithological Monographs 61: 1-13.
Boyle, W. A. 2008. Can variation in risk of nest predation explain altitudinal migration in tropical birds? Oecologia 155: 397-403.
Boyle, W. A. 2010. Does food abundance explain altitudinal migration in a tropical frugivorous bird? Canadian Journal of Zoology 88: 204-213.
Boyle, W. A., and C. J. Conway. 2007. Why migrate? A test of the Evolutionary Precursor Hypothesis. American Naturalist 169: 344-359.
Boyle, W. A., D. R. Norris, and C. G. Guglielmo. 2010. Storms drive altitudinal migration in a tropical bird. Proceedings of the Royal Society B 277: 2511–2519.
Briedis et al. 2020. Broad-scale patterns of the Afro-Palaearctic landbird migration. Global Ecology and Biogeography 29: 722-735.
Bruderer, B., and F. Liechti. 1998. Flight behaviour of nocturnally migrating birds in coastal areas – crossing or coasting. Journal of Avian Biology 29: 499-507.
Butler, C. J. 2003. The disproportionate effect of global warming on the arrival dates of short-distance migratory birds in North America. Ibis 145: 484-495.
Chan, K. 1994. Nocturnal activity of caged resident and migrant Silvereyes (Zosteropidae: Aves). Ethology 96: 313-321.
Chaves-Campos, J., J. E. Arévalo, and M. Araya. 2003. Altitudinal movements and conservation of Bare-necked Umbrellabird Cephalopterus glabricollis of the Tilarán Mountains, Costa Rica. Bird Conservation International 13: 45-58.
Chernetsov, N. 2006. Habitat selection by nocturnal passerine migrants en route: mechanisms and results. Journal of Ornithology 147: 185-191.
Chernetsov et al. 2025. Songbird migration between Eastern Europe and Southern Asia: how to deal with the arid belt? Journal of Ornithology 166: 291–296.
Chesser, R. T. 1994. Migration in South America, an overview of the austral system. Bird Conservation International 4: 91– 107.
Chesser, R. T. 2005. Seasonal distribution and ecology of South American Austral migrant flycatchers. In: Birds of two worlds (R. Greenberg and P. P. Marra, eds.), pp. 168-181. Johns Hopkins University Press, Baltimore, MD.
Coppack, T., S. F. Becker, and P. J. J. Becker. 2008. Circadian flight schedules in night-migrating birds caught on migration. Biology Letters 4: 619-622.
Coppack, T., and F. Pulido. 2009. Proximate control and adaptive potential of protandrous migration in birds. Integrative and Comparative Biology 49: 493-506.
Cox, G. W. 1985. The evolution of avian migration systems between temperate and tropical regions of the New World. American Naturalist 126:451–474.
Davis, L. A., E. H. Roalson, K. L. Cornell, K. D. McClanahan, and M. S. Webster. 2006. Genetic divergence and migration patterns in a North American passerine bird: implications for evolution and conservation. Molecular Ecology 15: 2141-2152.
Dawson, A. 2007. Seasonality in a temperate zone bird can be entrained by near equatorial photoperiods. Proceedings of the Royal Society B 274: 721-725.
Dingle, H. 2008. Bird migration in the southern hemisphere: a review comparing continents. Emu 108:341-359.
Dokter, A. M., F. Liechti, H. Stark, L. Delobbe, P. Tabary, and I. Holleman. 2010. Bird migration flight altitudes studied by a network of operational weather radars. Journal of the Royal Society Interface 8: 30–43.
Dufour et al. 2025. Ecological barrier crossing strategies in small migratory birds depend on wing morphology and plumage color. iScience 29: 114466.
Durant, J. M., D. Ø. Hjermann, G. Ottersen, and N. C. Stenseth. 2007. Climate and the match or mismatch between predator requirements and resource availability. Climate Research 33: 271-283.
Duriez, O., S. Bauer, A. Destin, J. Madsen, B. A. Nolet, R. A. Stillman, and M. Klaasen. 2009. What decision rules might Pink-footed Geese use to depart on migration? An individual-based model. Behavioral Ecology 20: 560-569.
Egevang, C., I. J. Stenhouse, R. A. Phillips, A. Petersen, J. W. Fox, and J. R. D. Silk. 2010. Tracking of Arctic Terns Sterna paradisaea reveals longest animal migration. Proceedings of the National Academy of Sciences USA 107: 2078-2081.
Eikenaar, C., and J. L. Schläfke. 2013. Size and accumulation of fuel reserves at stopover predict nocturnal restlessness in a migratory bird. Biology Letters 9: 20130712.
Falls, J. B. and J. G. Kopachena. 2010. White-throated Sparrow ( Zonotrichia albicollis). The Birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology, Ithaca, NY; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/128.
Fraser, K. C., A. W. Diamond, and L. Chavarría. 2010. Evidence of altitudinal moult- migration in a Central American hummingbird, Amazilia cyanura. Journal of Tropical Ecology 26: 645-648.
Fretwell, S. D. 1980. Evolution of migration in relation to factors regulating bird numbers. In: Migrant birds in the Neotropics (A. Keast and E. S. Morton, eds.), pp. 517-527. Smithsonian Institution, Washington, D.C.
Gauthreaux, S. A. 1982. The ecology and evolution of avian migration systems. In: Avian biology, vol. 6 (D. S. Farner and J. R. King, eds.), pp. 93-168. Academic Press, New York, NY.
Gerson, A. R., and C. G. Guglielmo. 2011. Flight at low ambient humidity increases protein catabolism in migratory birds. Science 333: 1434-1436.
Gill, R. E., Jr., T. L. Tibbitts, D. C. Douglas, C. M. Handel, D. M. Mulcahy, J. C. Gottschalck, N. Warnock, B. J. McCaffery, P. F. Battley, and T. Piersma. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proceedings of the Royal Society B 276: 447-457.
Gillis, E. A., D. J. Green, H. A. Middleton, and C. A. Morrissey. 2008. Life history correlates of alternative migratory strategies in American Dippers. Ecology 89: 1687-1695.
Gwinner, E. 1986. Circannual rhythms. Springer Verlag, Heidelberg, Germany.
Haines, A. M., M. J. McGrady, M. S. Martell, B. J. Dayton, M. B. Henke, and W. S. Seegar. 2003. Migration routes and wintering locations of Broad-winged Hawks tracked by satellite telemetry. Wilson Bulletin 115: 166-169.
Hatch, J. J. 2002. Arctic Tern ( Sterna paradisaea). The Birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology, Ithaca, NY; Retrieved from the Birds of North America Online: http:// bna.birds.cornell.edu/ bna/species/707.
Hau, M., M. Wikelski, and J. C. Wingfield. 1998. A Neotropical forest bird can measure the slight changes in tropical photoperiod. Proceedings of the Royal Society B 265:89-95.
Hedenström, A. 2010. Extreme endurance migration: what is the limit to non-stop flight? PLoS Biology 8(5): e1000362.
Helbig, A. J. 2003. Evolution of bird migration: a phylogenetic and biogeographic perspective. In: Avian migration (P. Berthold, E. Gwinner, and F. Sonnenschein, eds.), pp. 3-20. Springer, Berlin, Germany.
Helm, B., and E. Gwinner. 2006. Migratory restlessness in an equatorial nonmigratory bird. PLoS Biology 4: e110.
Horton, K. G., S. R. Morris, B. M. Van Doren, and K. M. Covino. 2023. Six decades of North American bird banding records reveal plasticity in migration phenology. Journal of Animal Ecology, doi.org/10.1111/136502656.13887.
Hunt, P. D., and B. C. Eliason. 1999. Blackpoll Warbler ( Dendroica striata). The Birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology, Ithaca, NY; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/431.
Ibáñez, C., J. Juste, J. L. Garcia-Mudarra, and P. T. Agirre-Medi. 2001. Bat predation on nocturnally migrating birds. Proceedings of the National Academy of Sciences USA 98: 9700-9702.
Jahn, A. E., D. J. Levey, J. A. Hostetler, and A. M. Mamani. 2010. Determinants of partial bird migration in the Amazon Basin. Journal of Animal Ecology 79: 983-992.
Jahn, A. E., D. J. Levey, and K. G. Smith. 2004. Reflections across hemispheres: a system-wide approach to New World bird migration. Auk 121: 1005-1013.
Jenkins, K. D., and D. A. Cristol. 2002. Evidence of differential migration by sex in White-throated Sparrows (Zonotrichia albicollis). Auk 119: 539-543.
Jones, T., and W. Cresswell. 2010. The phenology mismatch hypothesis: are declines in migratory birds linked to uneven global climate change? Journal of Animal Ecology 79: 98-108.
Joseph, L. 2005. Molecular approaches to the evolution and ecology of migration. In: Birds of two worlds: the ecology and evolution of migration (R. Greenberg and P. P. Marra, eds.), pp. 18-26. Johns Hopkins University Press, Baltimore, MD.
Karlsson, H., C. Nilsson, J. Backman, and T. Alerstam. 2011. Nocturnal passerine migrants fly faster in spring than in autumn: a test of the time minimization hypothesis. Animal Behaviour, online early.
Ketterson, E. D., and V. Nolan, Jr. 1976. Geographic variation and its climatic correlates in the sex ratio of eastern-wintering Dark-eyed Juncos (Junco hyemalis). Ecology 57: 679-693.
Ketterson, E. D., and V. Nolan, Jr. 1983. The evolution of differential bird migration. Current Ornithology 1: 357–402.
King, J. R., D. S. Farner, and L. R. Mewaldt. 1965. Seasonal sex and age ratios in populations of the White-crowned Sparrow of the race gambelii. Condor 67: 489-504.
Kingery, H. E. 1996. American Dipper ( Cinclus mexicanus). The Birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology, Ithaca, NY; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/229
Kjellén, N. 1994. Differences in age and sex ratio among migrating and wintering raptors in southern Sweden. Auk 111: 274-284.
Klaassen, R. H. G., T. Alerstam, P. Carlsson, J. W. Fox, and A. Lindstrom. 2011. Great flights by Great Snipes: long and fast non-stop migration over benign habitats. Biology Letters, early online.
Kokko, H. 1999. Competition for early arrival in migratory birds. Journal of Animal Ecology 68: 940-950.
Kokko, H., T. G. Gunnarsson, L. J. Morrell, and J. A. Gill. 2006. Why do female migratory birds arrive later than males? Journal of Animal Ecology 75: 1293-1303.
Laymon, S. A. 1989. Altitudinal migration movements of Spotted Owls in the Sierra Nevada, California. Condor 91: 837-841.
Lehikoinen, E., and T. H. Sparks. 2010. Changes in migration. In: Effects of climate change on birds (A. P. Møller, W. Fiedler, and P. Berthold, eds.), pp. 89-112. Oxford University Press, New York, NY.
Levey, D. J., and F. G. Stiles. 1992. Evolutionary precursors of long-distance migration: resource availability and movement patterns of Neotropical landbirds. American Naturalist 140: 447-476.
Liechti, F. 2006. Birds: blowin’ by the wind? Journal of Ornithology 147: 202-211.
Liechti, F., and B. Bruderer. 1998 . The relevance of wind for optimal migration theory. Journal of Avian Biology 29 : 561 – 568.
Lundberg, P. 1988. The evolution of partial migration in birds. Trends in Ecology and Evolution 3: 172–175.
Mackas, R. H., D. J. Green, I. B. J. Whitehorne, E. N. Fairhurst, H. A. Middleton, and C. A. Morrisey. 2010. Altitudinal migration in American Dippers (Cinclus mexicanus): do migrants produce higher quality offspring? Canadian Journal of Zoology 88: 369-377.
Marantz, C. A., and J. V. Remsen, Jr. 1991. Seasonal distribution of the Slaty Elaenia, a little-known austral migrant of South America. Journal of Field Ornithology 62: 162-172.
Marques, P. A. M., D. Sowter, and P. E. Jorge. 2010. Gulls can change their migratory behavior during lifetime. Oikos 119: 946-951.
Marquiss , M. 2002 . Common Crossbill . In: The migration atlas: movements of the birds of Britain and Ireland (C. V. Wernham , M. P. Toms , J. H. Marchant , J. A. Clark , G. M. Siriwardena , and S. R. Baillie , eds.), pp. 663-665. T. & A. D. Poyser , London , UK.
McKinnon, L., P. A. Smith, E. Nol, J. L. Martin, F. I. Doyle, K. F. Abraham, H. G. Gilchrist, R. I. G. Morrison, and J. Bêty. 2010. Lower predation risk for migratory birds at high latitudes. Science 327: 326-327.
Mills, A. M. 2005. Changes in the timing of spring and autumn migration in North American migrant passerines during a period of global warming. Ibis 147: 259-269.
Møller, A. P. 1994. Sexual selection and the Barn Swallow. Oxford University Press, Oxford, UK.
Møller, A. P., D. Rubolini, and E. Lehikoinen. 2008. Populations of migratory bird species that did not show a phonological response to climate change are declining. Proceedings of the National Academy of Sciences USA 105: 16195-16200.
Morbey, Y. E., and R. C. Ydenberg. 2001. Protandrous arrival timing to breeding areas: a review. Ecology Letters 4: 663-673.
Morten et al. 2025. Global Marine Flyways Identified for Long-Distance Migrating Seabirds From Tracking Data. Global Ecology and Biogeography 34: e70004.
Mrosovsky, N. 1978. Circannual cycles in hibernators . In: Strategies in the cold: natural torpidity and thermogenesis (L. C. H. Wang and J. W. Hudson, eds.), pp. 21 – 65. Academic Press, New York, NY.
Murphy-Klassen, H. M., T. J. Underwood, S. G. Sealy, and A. A. Czyrnyj. 2005. Long-term trends in spring arrival dates of migrant birds at Delta Marsh, Manitoba, in relation to climate change. Auk 122: 1130-1148.
Németh, Z., and F. R. Moore. 2007. Unfamiliar stopover sites and the value of social information during migration. Journal of Ornithology 148 (Suppl. 2): S369-S376.
Newton, I. 1972. Finches. Collins, London
Newton, I. 1995. Relationship between breeding and wintering ranges in Palaearctic-African migrants. Ibis 137: 241-249.
Newton, I. 2003a. The speciation and biogeography of birds. Academic Press, San Diego, CA.
Newton, I. 2003b. Geographical patterns in bird migration. In: Avian migration (P. Berthold, E. Gwinner, and E. Sonnenschein, eds.), pp. 211-224. Springer-Verlag, New York, NY.
Newton, I. 2006. Movement patterns of Common Crossbills Loxia curvirostra in Europe. Ibis 148: 782-788.
Newton, I. 2007a. Weather-related mass-mortality events in migrants. Ibis 149: 453-467.
Newton, I. 2007b. The ecology of bird migration. Academic Press, London, UK.
Newton, I. 2025. Migration mortality in birds. Ibis 167: 106–123.
Nystrom, K. G. K. 1997. Food density, song rate, and body condition in territory-establishing Willow Warblers (Phylloscopus trochilus). Canadian Journal of Zoology 75: 47 – 58.
Ogonowski, M. S., and C. J. Conway. 2009. Migratory decisions in birds: extent of genetic versus environmental control. Oecologia 161: 199-207.
Oring, L.W., and D. B. Lank. 1982. Sexual selection, arrival times, philopatry and sitefidelity in the polyandrous Spotted Sandpiper. Behavioral Ecology and Sociobiology 10: 185-191.
Outlaw, D. C., and G. Voelker. 2006. Phylogenetic tests of hypotheses for the evolution of avian migration: a case study using the Motacillidae. Auk 123: 455-466.
Outlaw, D. C., G. Voelker, B. Mila, and D. J. Girman. 2003. Evolution of long-distance migration in and historical biogeography of Catharus thrushes: a molecular phylogenetic approach. Auk 120: 299-310.
Parmelee, D. F. 1992. White-rumped Sandpiper (Calidris fuscicollis). The birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology, Ithaca, NY. Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/029.
Piersma, T., J. Pérez-Tris, H. Mouritsen, U. Bauchinger, and F. Bairlein. 2005. Is there a ‘migratory syndrome’ common to all migrant birds? Annals of the New York Academy of Sciences 1046: 282-293.
Piper, W. H., and R. H. Wiley. 1989. Correlates of dominance in wintering White-throated Sparrows: age, sex, and location. Animal Behaviour 37:298-310.
Popa-Lisseanu, A. G., A. Delgado-Huertas, M. G. Forero, A. Rodriguez, R. Arlettaz, and C. Ibáñez. 2007. Bats’ conquest of a formidable foraging niche: the myriads of nocturnally migrating songbirds. PLoS ONE 2(2): e205.
Pulido, F. 2007. The genetics and evolution of avian migration. BioScience 57: 165-174.
Rappole, J. H. 1995 . The ecology of migrant birds, a Neotropical perspective. Smithsonian Institution Press, Washington, D.C.
Rattenborg, N. C., B. H. Mandt, W. H. Obermeyer, P. J. Winsauer, R. Huber, M. Wikelski, and R. M. Benca. 2004. Migratory sleeplessness in the White-crowned Sparrow (Zonotrichia leucophrys gambelii ). PLoS Biology 2(7): e212.
Reynolds, J. D., M. A. Colwell, and F. Cooke. 1986. Sexual selection and spring arrival times of Red-necked and Wilson's phalaropes. Behavioral Ecology and Sociobiology 18: 303-310.
Richardson, W. J. 1990 . Timing and amount of bird migration in relation to weather: updated review. In: Bird migration: physiology and ecophysiology (E. Gwinner, ed.), pp. 78-101. Springer-Verlag, Berlin, Germany.
Rohwer, V. G., S. Rohwer, and J. H. Barry. 2008. Molt scheduling of western Neotropical migrants and up-slope movement of Cassin’s Vireo. Condor 110: 365-370.
Rubolini, D., A. G. Pastor, A. Pilastro, and F. Spina. 2002. Ecological barriers shaping fuel stores in Barn Swallows Hirundo rustica following the central and western Mediterranean flyways. Journal of Avian Biology 33: 15-22.
Rubolini, D., F. Spina, and N. Saino. 2004. Protandry and sexual dimorphism in trans-Saharan migratory birds. Behavioral Ecology 15: 592-601.
Rueda-Hernández, R., et al. 2023. Winter connectivity and leapfrog migration in a migratory passerine. Ecology and Evolution, https://doi.org/10.1002/ece3.9769
Saino, N., D. Rubolini, L. Serra, M. Caprioli, M. Morganti, R. Ambrosini, and F. Spina. 2010. Sex-related variation in migration phenology in relation to sexual dimorphism: a test of competing hypotheses for the evolution of protandry. Journal of Evolutionary Biology 23: 2054-2065.
Salewski, V., and B. Bruderer. 2007. The evolution of bird migration – a synthesis. Naturwissenschaften 94: 268-279.
Sapir, N., M. Wikelski, M. D. McCue, B. Pinshow, and R. Nathan. 2010. Flight modes in migrating European Bee-eaters: heart rate may indicate low metabolic rate during soaring and gliding. PLoS ONE 5(11): e13956.
Schaub, M., L. Jenni, and F. Bairlein. 2008. Fuel stores, fuel accumulation, and the decision to depart from a migration stopover site. Behavioral Ecology 19: 657-666.
Schmaljohann et al. 2022. Understanding the ecological and evolutionary function of stopover in migrating birds. Biological Reviews 97: 1231-1252.
Smith, R., I. Brown, and R. Mewaldt. 1969. Annual activity patterns of caged non-migratory White-crowned Sparrows. Wilson Bulletin 81: 419-440.
Somveille et al. 2013. Mapping Global Diversity Patterns for Migratory Birds. PLoS ONE 8: e70907.
Somveille et al. 2026. Climate, ecological dynamics, and the seasonal distribution of birds in mountains. Science Advances 12: DOI: 10.1126/sciadv.adz5547.
Sparks, T. H., K. Huber, R. L. Bland, H. Q. P. Crick, P. J. Croxton, J. Flood, R. G. Loxton, C. F. Mason, J. A. Newnham, and P. Tryjanowski. 2007. How consistent are trends in arrival (and departure) dates of migrant birds in the UK? Journal of Ornithology 148: 503-511.
Steadman, D. W. 2005. The paleoecology and fossil history of migratory landbirds. In: Birds of two worlds: the ecology and evolution of migration (R. Greenberg and P. P. Marra, eds.), pp. 5-17. Johns Hopkins University Press, Baltimore, MD.
Stiles, F. G. 1983. Birds. In: Costa Rican natural history (D. H. Janzen, ed.), pp. 502-530. University of Chicago Press, Chicago, IL.
Stotz, D. F. , J. W. Fitzpatrick, T. A. Parker III, and D. K. Moskovits. 1996. Neotropical birds: ecology and conservation . University of Chicago Press, Chicago, IL.
Strandberg, R., R. H. G. Klaassen, M. Hake, and T. Alerstam. 2010. How hazardous is the Sahara Desert crossing for migratory birds? Indications from satellite tracking of raptors. Biology Letters 6: 297–300.
Strandberg, R., R. H. G. Klaassen, P. Olofsson, and T. Alerstam. 2009. Daily travel schedules of adult Eurasian Hobbies (Falco subbuteo) – variability in flight hours and migration speed along the route. Ardea 97: 287-295.
Teitelbaumwe et al. 2023. Post-migratory nonbreeding movements of birds: A review and case study. Ecology and Evolution 13: e9893.
Tøttrup, A. P., K. Rainio, T. Coppack, E. Lehikoinen, C. Rahbek, and K. Thorup. 2010. Local temperature fine-tunes the timing of spring migration in birds. Integrative and Comparative Biology 50: 293-304.
Tulp, I., and H. Schekkerman. 2008. Has prey availability for Arctic birds advanced with climate change? Hindcasting the abundance of tundra arthropods using weather and seasonal variation. Arctic 61: 48-60.
Van Buskirk, J., R. S. Mulvihill, and R. C. Leberman. 2009. Variable shifts in spring and autumn migration phenology in North American songbirds associated with climate change. Global Change Biology 15: 760-771.
Végvári, Z., V. Bókony, Z. Barta, and G. Kovács. 2010. Life history predicts advancement of avian spring migration in response to climate change. Global Change Biology 16: 1-11.
Vergara, P., J. I. Aguirre, and M. Fernández-Cruz. 2007. Arrival date, age and breeding success in White Stork Ciconia ciconia. Journal of Avian Biology 38: 573-579.
Wikelski, M., L. B. Martin, A. Scheuerlein, M. T. Robinson, N. D. Robinson, B. Helm, M. Hau, and E. Gwinner. 2008. Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Philosophical Transactions of the Royal Society B 363: 411-423.
Winger, B. M. and T. M. Pegan. 2021. Migration distance is a fundamental axis of the slow-fast continuum of life history in boreal birds. Ornithology 138: 1–18.
Winkler et al. 2017. Long-Distance Range Expansion and Rapid Adjustment of Migration in a Newly Established Population of Barn Swallows Breeding in Argentina. Current Biology 27: 1080-1084.
Yong, D. L. et al. 2021. The State of Migratory Landbirds in the East Asian Flyway: Distributions, Threats, and Conservation Needs. Frontiers in Ecology and Evolution 9: 613172.
Yong, W., and F. R. Moore. 1993. Relation between migratory activity and energetic condition among thrushes following passage across the Gulf of Mexico . Condor 95: 934 - 943 .
Zink, R. M. 2002. Towards a framework for understanding the evolution of avian migration. Journal of Avian Biology 33: 433-436.
Züst et al. 2023. Pre-migratory flights in migrant songbirds: the ecological and evolutionary importance of understudied exploratory movements. Movement Ecology 11:78.