|
Ornithology Avian Mating Systems |
 Pied Avocets copulating. |
|
Ornithology Avian Mating Systems |
 Pied Avocets copulating. |
Birds exhibit a variety of mating systems:
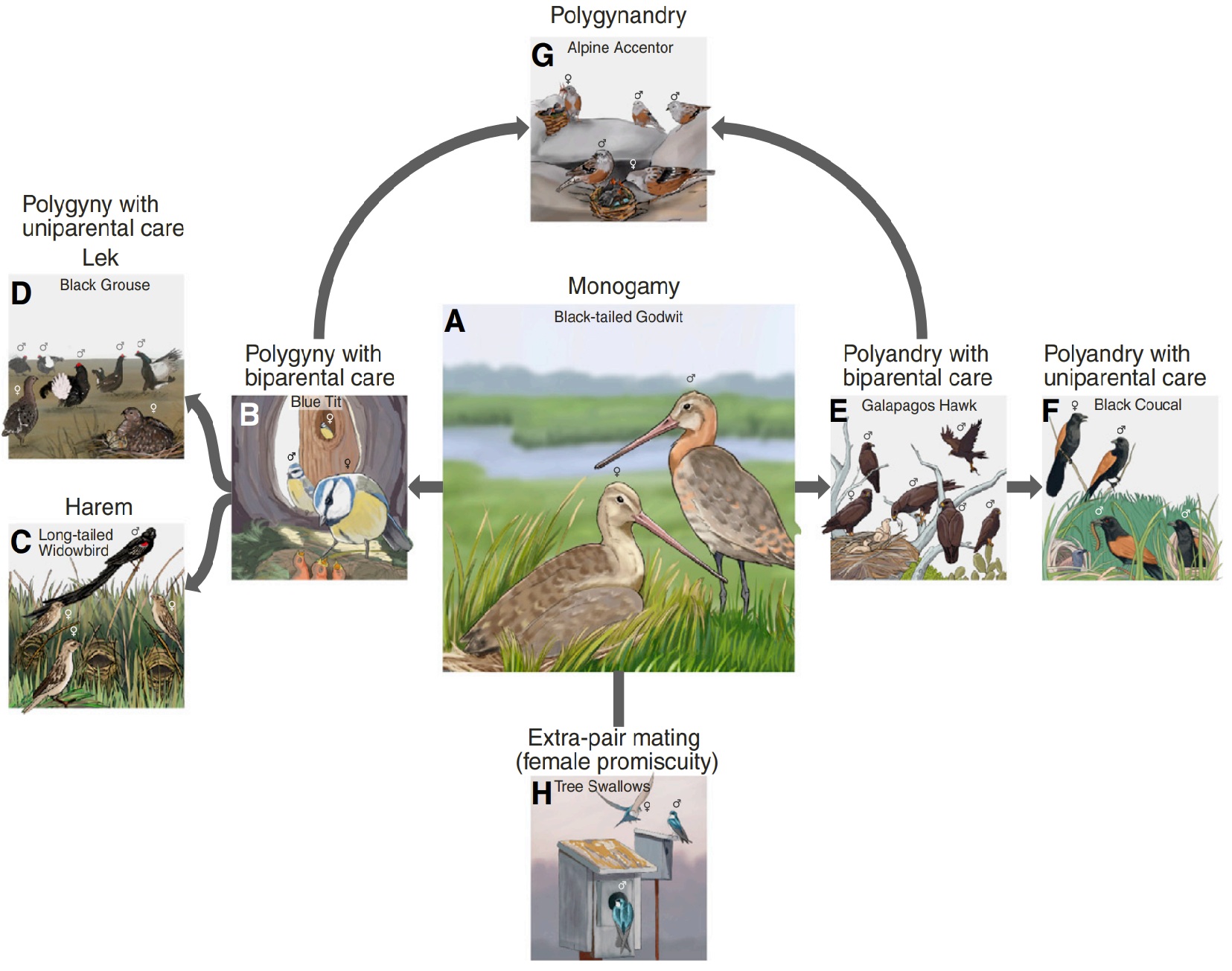
Examples of species of birds with different mating systems. (A) Social monogamy, the most common mating system that arose early
in the evolutionary history of the birds, is placed centrally. Additional social mates can lead to (B-D) social polygyny, (E,F) polyandry,
and (G) polygynandry. Additional copulation partners can lead to extra-pair or multiple paternity, independent of the social mating system (H).
At the extremes (C,D,F) are mating systems where only one parent incubates the eggs and cares for the young (From: Kempenaers 2022).
Polygynous Red-winged Blackbirds
Polyandrous Wilson's Phalaropes
| Seduced and abandoned - Seduced by a domineering, two-timing female and then abandoned to raise young that aren't his own, the male Wattled Jacana would seem to be a loser in the genetic lottery. But, at least some of the young he cares for carry his genes, and sometimes other males raise young that he has sired. Emlen et al. (1999) observed jacanas on Panama's Chagres River, witnessing more than 1,400 copulations between dominant females and much smaller males. Males were left to incubate eggs and raise chicks of uncertain paternity. Females, meanwhile, were copulating with other males. Jacanas are practically unique among vertebrate animals, in that females pair simultaneously with a harem of males (a mating system called polyandry) and the males care for the young. DNA fingerprinting revealed that more than 40% of jacana broods that males were tending included chicks fathered by a different male. However, when no extra males were available in a harem, all chicks were sired by the care-taking male. Thus a male's risk of being cuckolded comes entirely from other males that are simultaneously paired to the same female. "These results are surprising," said Emlen. "Biologists have assumed that males would only bear full parental responsibility for incubating eggs and raising the young under one condition: When there is certainty that the young are genetically his own -- that is, when the male's assurance of paternity is high. Instead, a male jacana sits on the nest, watching the mother of the chicks he will raise, while she continues to mate with other males nearby." Simultaneous polyandry is beneficial for female jacanas. "If a large, dominant female can maintain a harem," Emlen says, "her reproductive success is enhanced by having lots of males raising young for her. But the males may pay the cost. There thus is sexual conflict over the preferred mating system. Females benefit from pairing polyandrously; males, however, would avoid the cost of cuckoldry if they paired monogamously." Why does the male jacana tolerate this behavior by his mate? In heavily populated and highly competitive habitats with limited space for nests, some males never get the chance to reproduce at all. "I guess you could say the males are lucky to be seduced and abandoned, considering the alternative," Wrege, a co-author, says. "At least they're adding something of their own to the gene pool." | 
|
Monogamy:
White-necked Rockfowl (Picathartes gymnocephalus) mate for life
Divorce rate in monogamous birds increases with male promiscuity and migration distance. Socially monogamous birds may break up their partnership by a so-called ‘divorce’ behavior. Divorce rates vary immensely across avian taxa that have a predominantly monogamous social mating system. Although various factors associated with divorce have been tested, broad-scale drivers of divorce rate remain contentious. Chen et al. (2023) applied phylogenetic comparative methods to examine divorce rates from published studies of 186 species of birds from 25 orders and 61 families. The authors tested correlations between divorce rate and a group of factors, including promiscuity of both sexes (propensity to polygamy), migration distance, and adult mortality. Analysis revealed that only male promiscuity, but not female promiscuity, had a positive relationship with divorce rate. Further, migration distance was also positively correlated with divorce rate, whereas adult mortality rate exhibited no direct relationship with divorce rate. These findings indicated that divorce might not be a simple adaptive (by sexual selection) or non-adaptive strategy (by accidental loss of a partner) in birds, but could be a mixed response to sexual conflict and stress from the ambient environment. However, the number of species in our dataset was very limited, and our data did not include all major lineages of the avian tree of life. Given that social monogamy is ubiquitous in birds, a larger dataset of empirical studies and advanced theoretical modelling research will enhance our understanding of ecological drivers of divorce in birds.
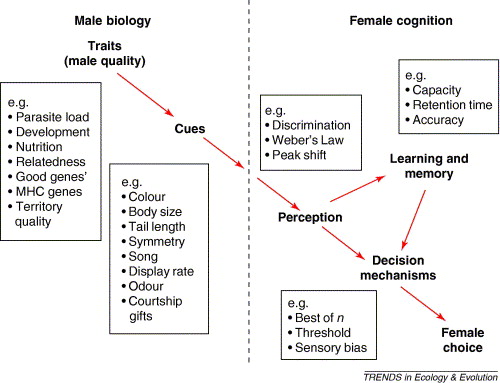
Female choice -- The flow of information from the male to the behavioral output of the female. The assumption is that male quality translates
into his traits,
which then serve as cues for the female. Her choice is based on her ability first to perceive the trait(s) of the male and then to use
that information to make her decision. The information from one male will be compared with that she has received from other males, and
this comparison will be affected by her cognitive abilities, including her memory, and her ability to process information from multiple sources
simultaneously (Bateson and Healy 2005).
Although the traits and cues of males mentioned above are probably known to most, some aspects of female perception and decision making may not
be as widely known. For example what is "Weber's law?" This 'law' basically says that the ability of an individual to discriminate a difference between
stimuli decreases with the size or intensity of those stimuli. So, for example, if females choose mates based, in part, on tail length, it's more difficult for
females to distinguish between males that differ slightly in tail length as the length of tails (over evolutionary time) increases. 'Peak shift' refers to the idea
that if a female acquires a 'preference' (e.g.,by imprinting on some characteristic or characteristics of her father), then, later in life, she may exhibit
a preference for males that exhibit an 'enhanced' version of that (or those) characteristic(s). As far as 'decision mechanisms', best-of-n means that a
females 'samples' a certain number of males (e.g., perhaps a migrant female arrives in a breeding area and visits five males and territories), then chooses
as her mate the 'best' of those sampled males. A 'threshold' means that a female may have a 'standard' in terms of mate quality and chooses as her mate
the first male that meets or exceeds that standard.
Molecular parallelism in signaling function across different sexually selected ornaments in a warbler - Extravagant ornaments are thought to signal male quality to females choosing mates, but the evidence linking ornament size to male quality is controversial, particularly in cases in which females prefer different ornaments in different populations. Here, we use whole-genome sequencing and transcriptomics to determine the genetic basis of ornament size in two populations of a widespread warbler, the Common Yellowthroat (Geothlypis trichas). Within a single subspecies, females in a Wisconsin population prefer males with larger black masks as mates, while females in a New York population prefer males with larger yellow bibs. Despite being produced by different pigments in different patches on the body, the size of the ornament preferred by females in each population was linked to numerous genes that function in many of the same core aspects of male quality (e.g., immunity and oxidative balance). These relationships confirm recent hypotheses linking the signaling function of ornaments to male quality. Furthermore, the parallelism in signaling function provides the flexibility for different types of ornaments to be used as signals of similar aspects of male quality. This could facilitate switches in female preference for different ornaments, a potentially important step in the early stages of divergence among populations (Abstract from Sly et al. 2022).
|
Evolution of molts and colorful plumage in songbirds by Jared Wolfe
Mechanisms of mate choice evolution. --- Several mechanisms have been put forward to explain mate choice:
The evolution of mate choice is based either on direct selection of a preference that gives a fitness advantage (mechanisms i–ii) or on indirect selection of a preference as it becomes genetically correlated with directly selected traits (mechanisms iii,iv). In addition, rather than favouring any particular display trait, mate choice might evolve because it conveys non-additive genetic benefits (mechanism v). These mechanisms are mutually compatible and can occur together, rendering the evolution of mating preferences a multiple-causation problem (From: Andersson and Simmons 2006).
Masked Bowerbird courtship
Northern Gannet courtship
Chilean Flamingos (Phoenicopterus chilensis)
 |
Female choice for male motor skills -- Sexual selection was proposed by Darwin to explain the evolution of male sexual traits such as ornaments and elaborate courtship displays. Empirical and theoretical studies have traditionally focused on ornaments; the reasons for the evolution of elaborate, acrobatic courtship displays remain unclear. Barske et al. (2011) addressed the hypothesis that females choose males on the basis of subtle differences in display performance, indicating motor skills that facilitate survival. Male Golden-collared Manakins (Manacus vitellinus) perform elaborate, acrobatic courtship displays. High-speed cameras were used to record the displays of wild males and analysed them in relation to male reproductive success. Females preferred males that performed specific display moves at greater speed, with differences of tens of milliseconds strongly impacting female preference. In additional males, heart rate was recorded telemetrically during courtship using miniature transmitters and analysis revealed that courtship is associated with profoundly elevated heart rates, revealing a large metabolic investment. These results provide evidence that females choose their mates on the basis of subtle differences in motor performance during courtship. Elaborate, acrobatic courtship dances may have evolved because they reflect motor skills and cardiovascular function of males.
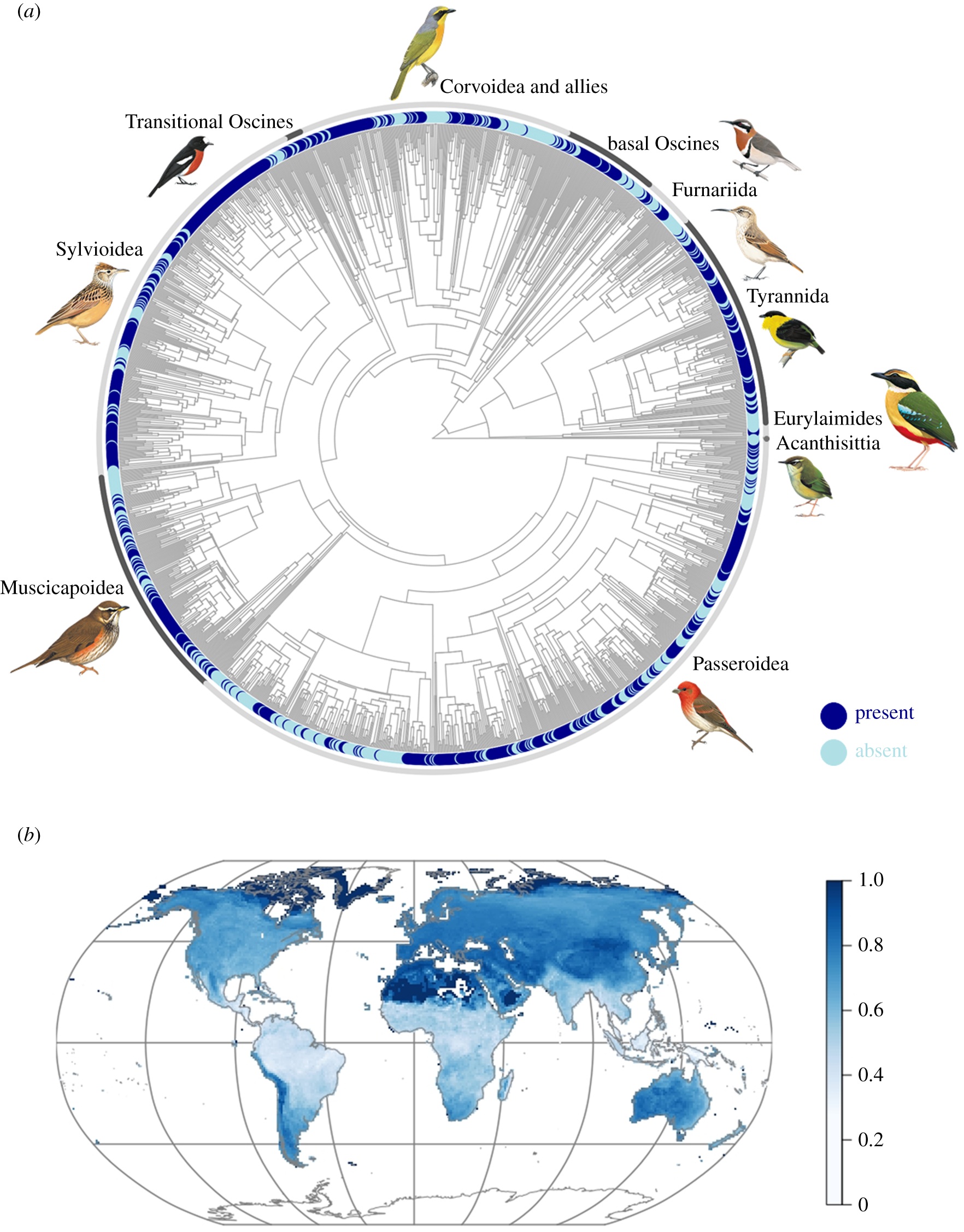
Distribution of aerial displays across passerines. (a) Distribution of aerial displays across a phylogeny of passerines
(1600 species). Highlighted are 10 major passerine groups with their representative species, starting with Acanthisittia (South
Island Wren) and followed by (going anticlockwise) Eurylaimides (African Pitta), Tyrannida (Golden-collared Manakin),
Furnariida (Slender-billed Miner), basal Oscines (Western Spinebill), Corvoidea and allies (Sulphur-breasted Bushshrike),
transitional Oscines (Norfolk Robin), Sylvioidea (Rufous-naped Lark), Muscicapoidea (Redwing), and Passeroidea
(Common Rosefinch). (b) Geographical distribution of aerial displays (1588 species) across passerine breeding assemblages.
Aerial displaying is a part of the sexual behaviour of several species of birds. Mikula et al. (2022) determined the presence and absence of aerial displays in males of 1732 species of passerine birds (Passeriformes) and tested for associations between aerial displays and a set of life-history and environmental predictors. Analysis revealed that species with males that perform aerial displays inhabited open rather than closed (forested) habitats. These species also exhibited higher levels of polygyny, had more elongated wings, migrated over longer distances, and had breeding ranges at higher latitudes. Aerial displaying was also associated with smaller body size and increased male plumage coloration. These results suggest that both sexual selection and natural selection have been important sources of selection on aerial displays in passerines.
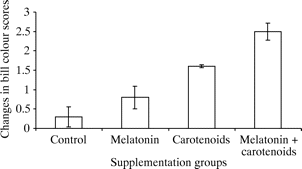 Changes in bill color scores of male Zebra Finches of different supplementation groups. Values are means ± 1 SE (N = 40). Changes were computed as the differences between the final and the initial values of bill color scores for each group (From: Bertrand et al. 2006). |
Carotenoids and antioxidants -- Carotenoid-based signals are thought to be indicators of male quality because they must be obtained from the diet and might thus indicate the ability of individuals to gather high-quality food. However, carotenoids are also known to have important physiological functions as immunoenhancers and antioxidants, and, as such, carotenoid-based sexual traits have also been suggested to reflect the health and antioxidant status of their bearers. This last idea is based on the hypothesis that carotenoids that are allocated to sexual signals are no longer available for the detoxification system. Recently, this hypothesis has been challenged on the grounds that antioxidant activity is not the main biological role of carotenoids. Instead, carotenoid-based sexual traits might signal the availability of other non-pigmentary antioxidant molecules that might protect carotenoids from free radical attacks and make them available for sexual advertisements. That is, because carotenoids are very sensitive to oxidation that alters and destroys their color, their signalling function can only be ensured if they are protected from the oxidation. Hartley and Kennedy (2004) suggested that only individuals that have a very effective antioxidant machinery (vitamins C and E, catalase, superoxide dismutase) would have enough unbleached carotenoids available for the sexual signal. Therefore, although carotenoid-based signals might still indicate the overall antioxidant status of their bearer, the mechanisms would be slightly different from the one originally envisaged. Bertrand et al. (2006) tested this hypothesis with Zebra Finches, a passerine species with a carotenoid-based signal: the color of the bill. They simultaneously manipulated the availability of carotenoids and of a non-pigmentary antioxidant (melatonin) in the drinking water. If the antioxidant properties of melatonin protect carotenoids from oxidation, birds supplemented with melatonin should have redder bills than birds not supplemented with melatonin, and birds supplemented with carotenoids and melatonin should have redder bills than birds supplemented with carotenoids alone. The findings of Bertrand et al. (2006) are in agreement with these predictions because carotenoid and melatonin supplementation had an additive effect on bill color. Thus, this is the first experimental evidence that a non-pigmentary antioxidant enhances the expression of a carotenoid-based sexual trait.
Polygyny:
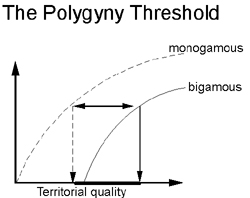
Predictions of the Polygyny Threshold model:

(Above) Wild male Little Bustard (left) visiting a male decoy (right) on an artificial lek;
(below) female Little Bustard visiting a male decoy (Photos by F. Jiguet).
Manipulating lek size and composition using decoys. A lek consists of clustered male territories that females visit strictly for the purpose of mating (there is no male parental care in lekking species). Male territories therefore do not hold resources attractive to females other than the males themselves. Leks are characterized by (i) male clustering; (ii) extreme bias in female choice, resulting in skewed male mating success; and, in many cases, (iii) stability of lek location over time. Four theoretical models have been proposed to account for the origin and maintenance of leks: hotspot, female preference, hotshot, and black hole models (described below). Each has been validated in particular cases, and most are not mutually exclusive; therefore, it has been difficult to contrast and separate them, empirically and experimentally. By using decoys to mimic natural leks in the Little Bustard, artificial leks attracted wild birds. Then, by manipulating artificial lek size and structure (sex ratio, male phenotype), the study of responses of wild males and females allowed Jiguet and Bretagnolle (2006) to test specific predictions derived from the four classical models of lek evolution. The hotspot model was not supported because female decoys did not attract wild males. Conversely, hotshot males do exist in this species (attracting both wild females and males), as does a female preference for a particular lek size (four males). Finally, males aggressive toward decoys attracted fewer females, consistent with one of the mechanisms by which the black hole model may work. Therefore, three models of lek evolution were partly or fully supported by our experimental results: hotshot, female preference, and black hole models. We suggest that these models actually fit within each other, ensuring the evolution, functioning, and long-term maintenance of leks.
Male Little Bustard call and display
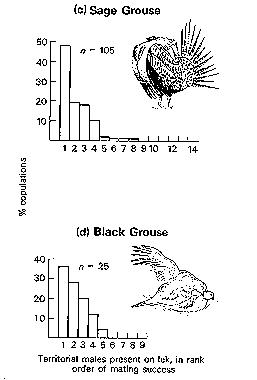
Male dominance polygyny:
Natural and sexual selection
Male displaying at White-collared Manakin lek in Costa Rica
Courtship displays and mating at a Marvelous Spatuletail hummingbird lek in Peru
Lesser Bird of Paradise (Paradisaea minor): At Lek Arena
Greater Sage-Grouse lek

Examples of bowerbird bowers. Top row = Avenue-builders; Bottom row = Maypole-builders
The bowerbirds in New Guinea and Australia include species that build the largest and perhaps most elaborately decorated constructions outside of humans. Males use these courtship bowers, along with their displays, to attract females. In these species, the mating system is polygynous and females alone build nests, incubate eggs, and feed nestlings. Males in one
bowerbird clade build ‘avenue’ style bowers – typically consisting of two parallel walls constructed using sticks and dry grasses – whereas species of another clade build ‘maypole’ style bowers that are usually characterized by a central, spire-like structure. Male bowerbirds spend considerable time and energy on constructing and decorating their bowers and courts with colorful objects and plant material, every species has its own preferred objects and color. In some species, males clear a court of several square-meters for
their elaborate displays.
The evolution of the elaborate breeding behavior of the polygynous bowerbirds is driven by female mate selection. Females visit and inspect several bowers and male displays before making their choice.
The evolution of bower building is largely unclear, although it has been assumed that bowers function as a replacement or extension of male plumage ornaments. Another hypothesis for the evolution of bower-building is that maypoles and avenues provide protection for females against unwanted mating. Males typically display just outside the bowers and females observe them from
inside the bowers. This is also where mating takes place if females accept the male, but if a male tries approach a female who chooses not to mate she can easily escape before the male comes close (From: Ericson et al. 2020).
What conditions favor the evolution of leks?
Sharp-tailed Grouse lek
Club-winged Manakin displaying
Lance-tailed Manakin display
| Development of coordinated singing in cooperatively displaying Long-tailed Manakins -- Long-tailed manakins (Chiroxiphia linearis) have a puzzling social system in which teams of two males display cooperatively in dispersed lek arenas, but only the alpha partner mates with visiting females. One benefit of performing as a nonmating partner might be to gain experience as an "apprentice" to improve the performance of the complex duet song and joint dance. Trainer et al. (2002) examined the relationship between the age of singers and two measures of singing performance: song variability and sound frequency matching. Singing performance improved with age; variability in four song characteristics of males less than 3 years old was greater than that in their older partners, and frequency matching increased with the age of the younger partner. These results and evidence from long-term monitoring of banded birds best support the hypothesis that frequency matching develops over several years during the complex and protracted process of partner formation. Nonmating males may benefit from increasing their competence at display, eventually enjoying increased mating success when they inherit display sites from older males.. |  |
Female mimics in a lekking shorebird -- Female mimics are known from many species, but permanent, non-conditional, alternative mating strategies are only known from an isopod, a fish, a lizard and a bird. The single bird example refers to lek-breeding Ruffs (Philomachus pugnax), a shorebird for which two strategies (independent and satellite) have been known for over 50 years. Ruffs also provided the single case of an animal with two, rather than three, permanent alternative mating strategies. Jukema and Piersma (2006) described a rare female-like morph of Ruffs: the ‘missing’ third alternative mating strategy that they called ‘faeder.’ Faeders are slightly larger than females and have testes 2.5 times the size of testes of normal males. On leks in aviaries and in the wild, they appear to combine feminine and masculine behaviors. Faeders may represent the ancestral, care-giving, male strategy, but their relatively large testes suggest that currently they behave as sneakers.
(a) A female mimic (or faeder), (b) on the left are two female mimics, on the right a typical male, and between them a single female, (c) a female mimic being mounted by a male [Photos by J. and T. Champion (a & c) and Y. Verkuil (b)]. |
 |
Polyandry:
Other factors potentially important in the evolution of polyandry:
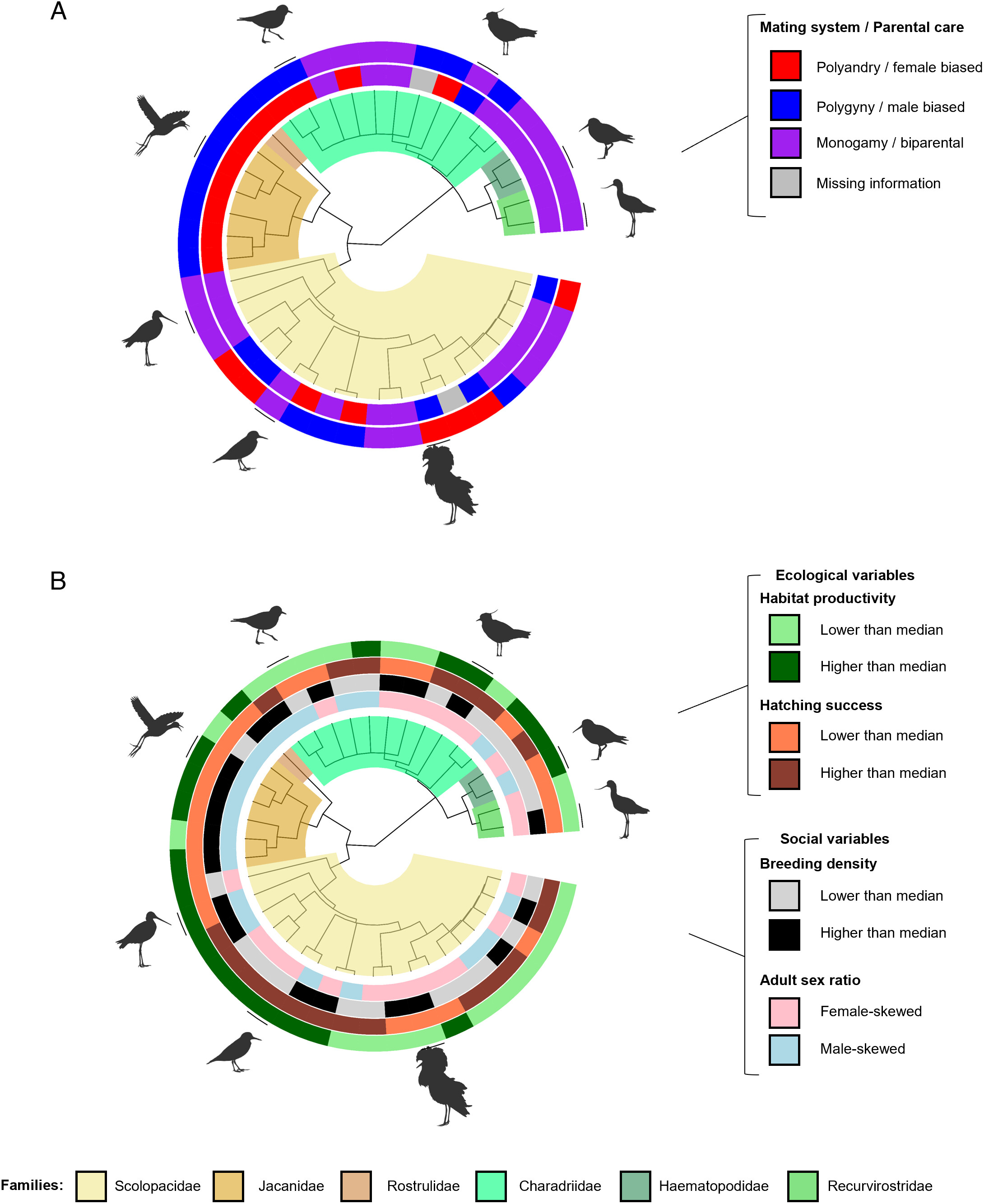
Distribution of (A) mating systems and parental care and (B) ecological and social variables in 41 species of shorebirds.
(A) Colored circles show 1) parental care score bias (outer circle) and 2) polygamy bias (inner circle). The latter variable
is represented either by polygamy frequency bias (most species) or by polygamy score bias (with three species with no
polygamy frequency data). (B) Colored circles show 1) habitat productivity (outer circle, light and dark green), 2) hatching
success (second circle, light and dark brown), 3) breeding density (third circle, gray and black), and 4) adult sex ratio (inner
circle, pink and blue). Light and dark colors represent an average value for the species lower or higher, respectively, than the
median in habitat productivity, hatching success, and breeding density. For adult sex ratio, pink and blue colors represent an
average value for the species lower or higher, respectively, than 0.5 (i.e., even sex ratio). Silhouette images represent from bottom
following clockwise direction: Ruff (Calidris pugnaxa), Common Sandpiper (Actitis hypoleucos), Black-tailed Godwit (Limosa limosa),
Northern Jacana (Jacana spinosa), Kentish Plover (Charadrius alexandrinus), Northern Lapwing (Vanellus vanellus), Eurasian
Oystercatcher (Haematopus ostralegus), and American Avocet (Recurvirostra americana) (From: Fresneau et al. 2024).
Extra-pair copulations
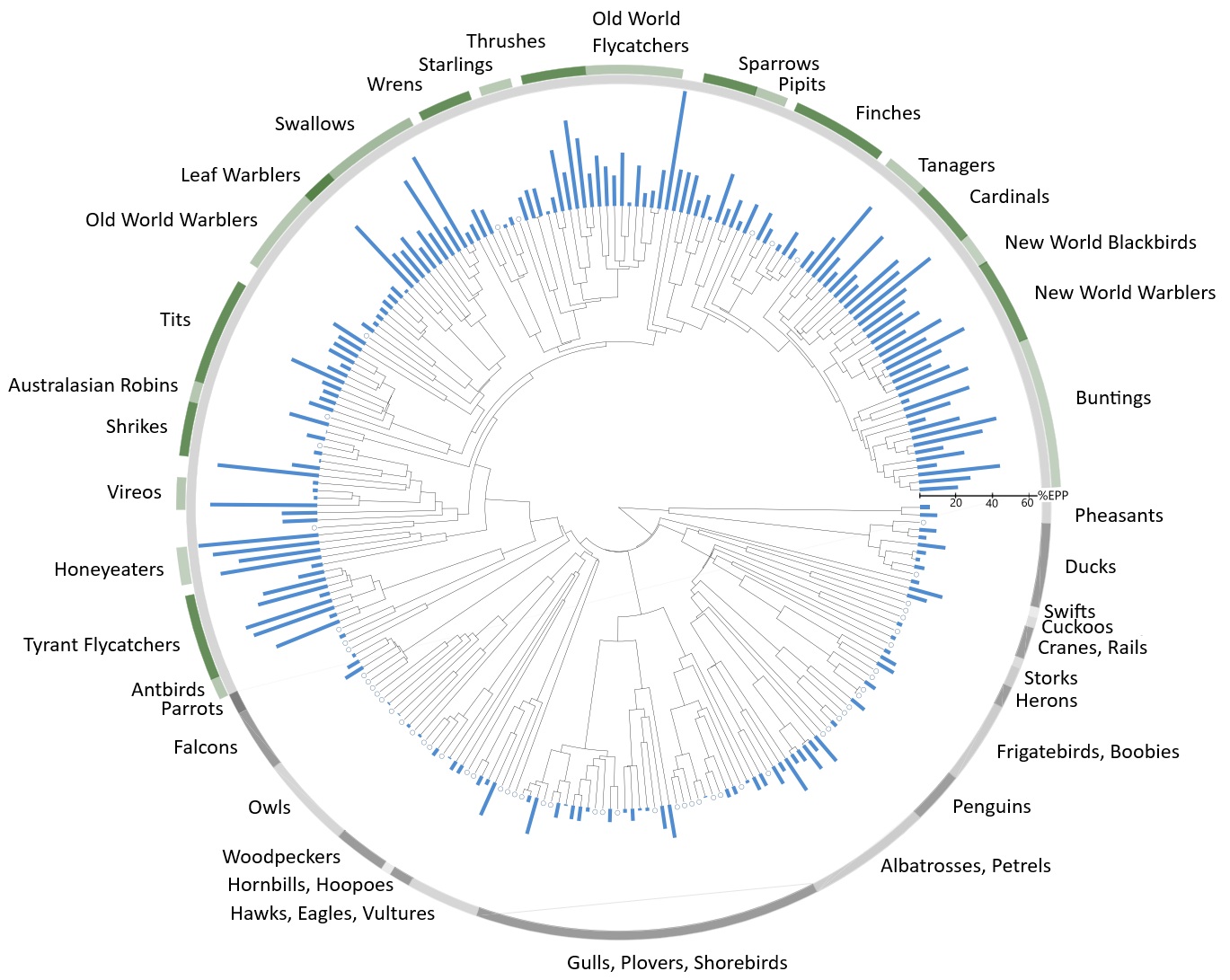
Average proportion of EPP (blue bars) for all sampled socially monogamous bird species, with
zero EPP indicated by an empty circle (From: Brouwer and Griffith 2019).
Considering all avian species for which genetic polyandry has
been determined, extra-pair offspring have been detected in 75% of the 342 sampled species. However, avian species have different breeding systems, categorized by the social relationships between males and females within a population during reproduction, and particularly the pattern of parental care. The most prevalent mating system in birds is
social monogamy with biparental care (81% of
species). EPP has been found reported in 76% of these socially monogamous
species. However, the highest reported EPP rates have been found
in cooperatively breeding speciesm e.g., 81.4% of offspring in Australian
Magpies and 71.8% in Superb Fair-wrens. Comparing the distributions of EPP rates shows that
there are proportionally more cooperatively breeding species with
EPP levels >30% (20% of cooperatively breeding species)
than those socially monogamous species with biparental care (14%
of socially monogamous species). The relatively high incidence of extra-pair paternity in co-operatively breeding birds makes sense because the
presence of subordinate helpers might reduce the costs for females to engage in extra-pair mating, because helpers can compensate for the reduced investment in parental care by males
when they suspect they have been cuckolded (From: Brouwer and Griffith 2019).
Because, in most populations, many individuals do not engage in EPCs, such behavior must also have costs:
Female Great Horned Owl fed by male (courtship feeding)
To maximize their own fitness (reproductive success), males try to prevent their mates from engaging in EPCs. From an evolutionary perspective, it simply doesn't pay to be cuckolded (that is, having a mate engage in EPCs, and end up caring for another male's offspring). To avoid being cuckolded, males may use paternity guards such as:
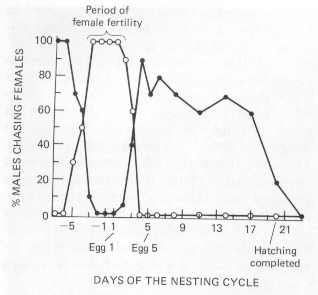 |
Mate guarding by male Bank Swallows. Males chase other females several days before their mates begin egg-laying (when their mates are not fertile). When their mates are fertile (within a few days of laying their first egg), males chase their own females almost exclusively. Then, once egg-laying is nearly finished (and mates are no longer fertile), males again chase other females. Open circles = males chasing a mate; closed circles = males chasing females other than their mate (From Beecher and Beecher 1979). |
Why is there so much variation among &
within species in mating strategies?
Large brain size is associated with low extra-pair paternity across bird species - : Gaining extrapair copulations (EPCs) is a complicated behavior process.
The interaction between males and females to procure EPCs may be involved in brain
function evolution and lead to a larger brain. Thus, Chen et al. (2021) hypothesized that extrapair
paternity (EPP) rate can be predicted by relative brain size in birds. Past work has
implied that the EPP rate is associated with brain size, but empirical evidence is rare.
Chen et al. (2021) collated data from published references on EPP levels and brain size of
215 bird species to examine whether the evolution of EPP rate can be predicted by
brain size using phylogenetically generalized least square (PGLS) models and phylogenetic path analyses.
EPP rates (both the percentage EP offspring and percentage
of broods with EP offspring) were found to be are negatively associated with relative brain size. Phylogenetic path analysis was applied to test the causal relationship between relative brain
size and EPP rate. Best-supported models (ΔCICc < 2) suggested that large brains lead to a reduced EPP rate, which failed to support the hypothesis that high rates of EPP cause the evolution of larger brains. The results of this study indicates that pursuing EPCs may be a natural instinct in birds and the interaction between males and females for EPCs may lead to large brains, which in turn may restrict their EPC level for both sexes across bird species. |
Neodorf, Diane L. H. 2004. Extrapair paternity in birds: understanding variation among species. Auk 121: 302-307:
Hypothesis to Explain Variation in Extrapair Fertilizations among Species

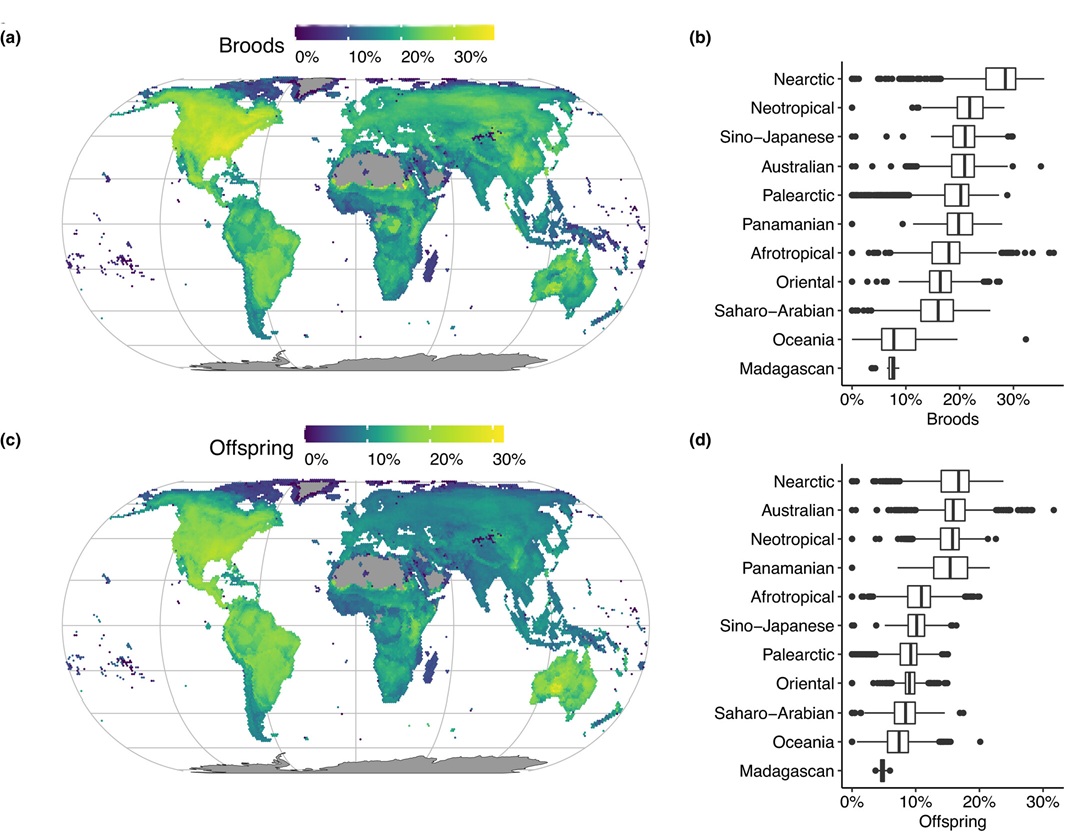
Geographical variation in the proportions of extra-pair broods (a, b) and extra-pair offspring (c, d).
Variation among zoogeographical realms (b,d) is indicated with box plots (From: Valcu et al. 2021).

Female extrapair behavior can evolve via indirect selection on males -- In many species that form socially monogamous pair bonds, a considerable proportion of the offspring is sired by extrapair males. This observation has remained a puzzle for evolutionary biologists: although mating outside the pair bond can obviously increase the offspring production of males, the benefits of such behavior to females are less clear, yet females are known to actively solicit extrapair copulations. For more than two decades adaptionist explanations have dominated the discussions, yet remain controversial, and genetic constraint arguments have been dismissed without much consideration. An intriguing but still untested hypothesis states that extrapair mating behavior by females may be affected by the same genetic variants (alleles) as extrapair mating behavior by males, such that the female behavior could evolve through indirect selection on the male behavior. Forstmeier et al. (2011) found that in socially monogamous Zebra Finches, individual differences in extrapair mating behavior have a hereditary component. Intriguingly, this genetic basis is shared between the sexes, as shown by a strong genetic correlation between male and female measurements of extrapair mating behavior. Hence, positive selection on males to sire extrapair young will lead to increased extrapair mating by females as a correlated evolutionary response. This behavior leads to a fundamentally different view of female extrapair mating: it may exist even if females obtain no net benefit from it, simply because the corresponding alleles were positively selected in the male ancestors.
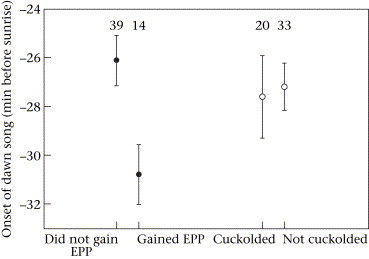
Mean ± SE onset of dawn song in Blue Tits for (a) males that did or did not gain
extrapair paternity (EPP) and (b) males that lost paternity (cuckolded) or did not lose
paternity in their own nest.
Sample sizes are shown above data points.
Early birds are sexy -- Sexual selection theory predicts that signals reflecting the relative quality of individuals should be used in mate choice. Females could base their choice of copulation partners on male secondary sexual traits that honestly signal male age, as predicted by the age-based indicator mechanism. Studies have shown that female blue tits prefer older males and that aspects of dawn song reflect male quality, but it remains unknown whether dawn song characteristics correlate with male age. Poesel et al. (2006) compared dawn song characteristics of second-year (SY) and older (ASY) male Blue Tits, and tested for age-related changes within individuals and differential overwinter survival of SY males. They also investigated the relation between dawn song and paternity gain and loss, and found that ASY male Blue Tits began to sing earlier relative to sunrise than did SY males. This difference in the onset of dawn singing was due to age-related changes in individual performance rather than differential survival of individuals with varying expression of the trait. Males that began to sing earlier at dawn had more mating partners, and were more likely to gain extrapair paternity. These findings suggest that the onset of dawn song can provide a simple mechanism for females to assess the relative quality of their mate and of neighbouring males. Female Blue Tits appear to use the onset of singing as a cue for their choice of extrapair partners.
Blue Tit nest
Brood parasitism:

Broods of mixed maternity, like this one, are common in Common Goldeneye and arise either through conspecific brood parasitism or amalgamation of broods after hatching. The ducklings with different cheek colors were color marked at different nest boxes and thus originated from the nests of different females (photograph by B. Lyon; Lyon and Eadie 2000). |
| Family matters: Kin selection and conspecific brood
parasitism -- Conspecific brood parasitism as
an alternative female breeding tactic is particularly common in ducks,
where hosts often receive eggs laid by parasitic females of the same species
and raise their offspring. Andersson and Åhlund (2000) tested several
aspects of a kin selection explanation for this phenomenon in Common
Goldeneyes (Bucephala clangula) by using techniques of egg albumen
sampling and statistical bandsharing analysis. They found that hosts and
primary parasites were often related, with mean r = 0.13, about as high
as between first cousins. Relatedness to the host was higher in nests where
a parasite laid several eggs than in those where she laid only one. Returning
young females parasitized their birth nestmates (social mothers or sisters,
which are usually also their genetic mothers and sisters) more often than
expected by chance. Such adult relatives were also observed together in
the field more often than expected and for longer periods than other females.
Relatedness and kin discrimination, which can be achieved by recognition
of birth nestmates, therefore play a role in these tactics and probably
influence their success.
Andersson and Åhlund's (2000) findings are exciting because conspecific brood parasitism has been viewed traditionally as an interaction that squarely pits the interests of the "parasite" against those of the host, leading perhaps to escalating coevolutionary arms races between them. If, however, parasites and hosts are related, the argument reverses and what appeared to have been a parasitic exchange becomes, instead, a cooperative one not dissimilar from other well-known cooperative breeding systems (Lyon and Eadie 2000). |

(Correction: cowbirds are in the family Icteridae, not Fringillidae)
;
Brood Parasites of Africa
Learned use of an innate sound-meaning association in birds
Signals in vocal communication systems range from innate to learned. Although innate and learned signals are often assumed to be independent, Darwin speculated that they could be evolutionarily related, with the former being the foundation of the latter even in our own communication system, language. Feeney et al. (2025) tested this hypothesis by studying the vocal communication systems of avian hosts of brood parasites. They found that 21 bird species separated by approximately 53 million years of evolution produce structurally similar ‘whining’ vocalizations towards their respective brood parasites. Exploring the social correlates of whining vocalization production, they found that species that produce this vocalization often exist in areas with dense parasite–host networks, suggesting that its production facilitates interactions among host species. Experiments across three continents show that this vocalization is referential towards brood parasites in multiple host species, that hearing them elicits an innate rapid recruiting response, and that host species from different continents respond equally to the whining vocalizations of each other, indicating that convergent use facilitates cooperative defences across species. These results provide an example of a referential animal vocalization for which sound production in the correct context is learned but for which hearing it elicits an innate response, representing an intermediate between innate and learned signals.
Eavesdropping as a Frontline Defense Against Brood Parasitism
Cuckoos and their hosts -- The Old World family Cuculidae contains about 50 obligatory parasites, some of which are host generalists and some specialists. The Common Cuckoo (Cuculus canorus) has an especially interesting pattern of host use: although it parasitizes over 100 species across its range, in any one locality only a few species are parasitized, and most individual female cuckoos use only one host species. All the female cuckoos parasitizing one host species are referred to as a gens (plural, gentes). Furthermore, some gentes lay eggs that mimic those of their host. A mechanism for the maintenance of this odd state of affairs was proposed a century ago: maternal inheritance of egg type combined with imprinting of the juvenile cuckoo on her host. However, there is still no evidence to support this hypothesis (Winfree 1999).
Cowbird hosts and cowbirds --In contrast to the cuckoo, the cowbird is a generalist at the level of both the species and the individual. Compared with the 50 species of parasitic cuckoos, there are only five species of parasitic cowbirds. There is no evidence that cowbirds lays eggs mimetic of its hosts' eggs. However, many cowbird hosts accept these nonmimetic eggs. Cuckoos have been brood parasites much longer (more than 60 million years) than cowbirds (2.8–3.8 million years) (Winfree 1999).

Common Cuckoo
(http://www.nikon.co.jp)
Evolution of brood parasitism in the Common Cuckoo
On an evolutionary scale, if parasites benefit from brood parasitism and hosts are harmed by it, why do hosts tolerate parasitism? Hosts can get rid of the parasitic egg in various ways, including ejecting the egg from the nest with their bill, building an additional layer of nest lining over the unwanted egg or abandoning the parasitized nest. There are two primary hypotheses to explain why rejection is not universal among hosts of brood parasites:
Female Brown-headed Cowbird laying an egg
Chick killing by brood parasitic honeyguides -- The most virulent avian brood parasites obligately kill host young soon after hatching, thus ensuring their monopoly of host parental care. While the host eviction behavior of cuckoos (Cuculidae) is well documented, the host killing behavior of honeyguide (Indicatoridae) chicks has been witnessed only once, 60 years ago, and never in situ in host nests. Spottiswoode and Koorevaar (2012) described the first detailed observations of honeyguides killing host chicks with their specially adapted bill hooks, based on repeated video recordings. Adult Greater Honeyguides (Indicator indicator) puncture host eggs when they lay their own, but in about half of host nests at least one host egg survived, precipitating chick killing by the honeyguide hatchling. Hosts always hatched after honeyguide chicks, and were killed within hours. Despite being blind and in total darkness, young honeyguides attacked host young with sustained biting, grasping and shaking motions. Attack time of 1–5 min was sufficient to cause host death, which took from 9 min to over 7 hr from first attack. Honeyguides also bit unhatched eggs and human hands, but only rarely bit the host parents feeding them.
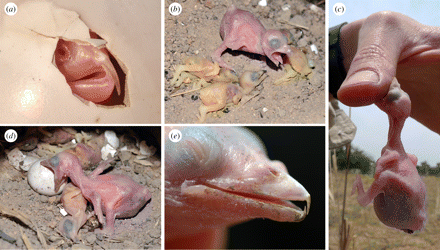
(a) Hatching Lesser Honeyguide, showing fully developed bill hooks; (b) Greater Honeyguide chick with three recently
killed little bee-eater hatchlings; (c) biting human hand; (d) biting unhatched Swallow-tailed Bee-eater egg; (e) aged about 8 days.

An African Village Weaverbird at a partially constructed nest.
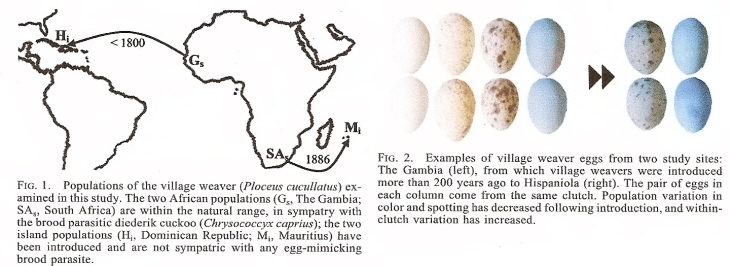
Figures 1 and 2 from Lahti (2006).
Evolution of bird eggs in the absence of cuckoo parasitism -- Historical introductions of species into new habitats can create rare opportunities to test evolutionary hypotheses, such as the role of natural selection in maintaining traits. Lahti (2005) examined two independent introductions of the African Village Weaverbird (Ploceus cucullatus) to islands where selection on egg appearance traits is expected to differ markedly from that of the source populations. The color and spotting of village weaver eggs in Africa are highly consistent within clutches, but highly variable between individuals. These two features may be an evolutionary response to brood parasitism. In Africa, weavers are parasitized by each other and by the Diederik Cuckoo (Chrysococcyx caprius), an egg mimic. African Village Weavers were introduced one century ago to Mauritius, and over two centuries ago to Hispaniola. Both islands are devoid of egg-mimicking brood parasites. In these two populations, between-individual variation and within-clutch consistency in egg appearance have both decreased, as has the incidence of spotting, relative to the source populations in Africa. These reductions are more pronounced on Hispaniola, the earlier introduction. Such changes support the hypothesis that egg appearance in the African Village Weaver has been maintained by natural selection as a counteradaptation to cuckoo brood parasitism. These results illustrate that the removal of an agent of selection can sometimes bring about rapid evolutionary consequences.

Female Brown-headed Cowbird removing an egg from a meadowlark
nest
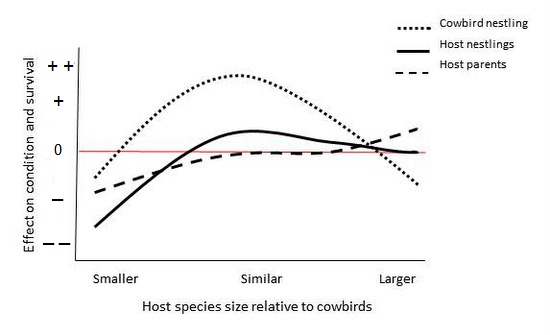
Generalist brood parasites parasitize host species that can vary dramatically in size. For example, Brown-headed Cowbirds (adult mass about 44 grams) parasitize species ranging in size from gnatcatchers (Polioptila spp.; about 5 grams) to meadowlarks (Sturnella spp.; some > 100 grams). As a result, the size of nestling cowbirds relative to host nestlings varies considerably, and these differences can influence both the growth and likelihood of fledging of young hosts and cowbirds as well as the condition of host parents. In general, young cowbirds tend to do best with host species of similar size, do less well with very small host species because very small host parents are limited in the amount of food they can provide (although this may vary depending on the particular host species), and fair worst as host species increase in size (because larger host nestlings can outcompete smaller cowbird nestlings). The presence of a cowbird nestling can also affect host nestlings, with very small hosts outcompeted by the cowbird and so less likely to survive, similar-sized nestlings possibly benefitting by increased provisioning rates resulting from the begging of the young cowbird, and larger nestlings generally little impacted by the cowbird’s presence (Figure below). Impacts of host parents similar in size to cowbirds are likely minimal, whereas very small host parents may provision at higher-than-normal rates at some expense to their condition and vary large host parents possibly benefitting because a young cowbird may not need as much food as their own nestlings.
Young
cowbirds join nestmates in making noise, then hog the food -- Young Brown-headed Cowbirds join nestmates in a begging chorus that brings
in more food than one noisy cowbird chick could demand from its host parents.
By eating  more
than their share, Kilner et al. (2004) found that cowbird chicks actually
grow faster when sharing the nest and food with two host chicks than when
alone in the nest. "The cowbird alone is incapable of bringing in enough
parental resources - basically food - to be able to grow optimally," said
co-author Mark Hauber. "When it has nestmates, the whole nest brings in
more parental care, because there is more begging altogether, and so the
parents attend the nest more. But the cowbird monopolizes the feeding attempts
by the parents. In our experiments, instead of getting 33% of the feedings
that a brood of two host chicks and one cowbird chick gets, the cowbird
actually got over 50% of the feeding. So, it grew better than when it was
alone." Kilner et al. (2004) switched eggs among Eastern Pheobe nests to
test various scenarios. They added a single cowbird egg to 20 nests, and
after the chick hatched, they removed the host eggs from 10 of those. In
the other 10 nests, after the cowbird hatched they placed two phoebe hatchlings
of the same age or a day older. They then monitored the nests with video
cameras to determine how often each chick ate, and daily for nine days
weighed each chick. The researchers found that by day 8, cowbird chicks
raised with two phoebe chicks were, on average, 14% heavier than cowbird
chicks raised alone. Also, phoebe parents brought food to nests with three
hatchlings about four times each hour, versus 1.5 times per hour for a
lone cowbird chick. This strategy of sharing the nest to gain more resources
appears to be successful generally among all the 100 or so species of birds
parasitized by cowbirds, Hauber said. -- By Robert Sanders more
than their share, Kilner et al. (2004) found that cowbird chicks actually
grow faster when sharing the nest and food with two host chicks than when
alone in the nest. "The cowbird alone is incapable of bringing in enough
parental resources - basically food - to be able to grow optimally," said
co-author Mark Hauber. "When it has nestmates, the whole nest brings in
more parental care, because there is more begging altogether, and so the
parents attend the nest more. But the cowbird monopolizes the feeding attempts
by the parents. In our experiments, instead of getting 33% of the feedings
that a brood of two host chicks and one cowbird chick gets, the cowbird
actually got over 50% of the feeding. So, it grew better than when it was
alone." Kilner et al. (2004) switched eggs among Eastern Pheobe nests to
test various scenarios. They added a single cowbird egg to 20 nests, and
after the chick hatched, they removed the host eggs from 10 of those. In
the other 10 nests, after the cowbird hatched they placed two phoebe hatchlings
of the same age or a day older. They then monitored the nests with video
cameras to determine how often each chick ate, and daily for nine days
weighed each chick. The researchers found that by day 8, cowbird chicks
raised with two phoebe chicks were, on average, 14% heavier than cowbird
chicks raised alone. Also, phoebe parents brought food to nests with three
hatchlings about four times each hour, versus 1.5 times per hour for a
lone cowbird chick. This strategy of sharing the nest to gain more resources
appears to be successful generally among all the 100 or so species of birds
parasitized by cowbirds, Hauber said. -- By Robert Sanders |
 Prothonotary Warbler nest parasitized by a cowbird (Courtesy PNAS). |
 Male Prothonotary Warbler (Protonotaria citrea) at a nest (Photo by Jeff Hoover). |
Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs -- Why do many hosts accept costly avian brood parasitism even when parasitic eggs and nestlings differ dramatically in appearance from their own? Scientists argue that evolutionary lag or equilibrium can explain this evolutionary enigma. Few, however, consider the potential of parasitic birds to enforce acceptance by destroying eggs or nestlings of hosts that eject parasitic eggs and thereby reject parasitism. This retaliatory "mafia" behavior has been reported in one species of parasitic cuckoo but never in parasitic cowbirds. Hoover and Robinson (2007) obtained experimental evidence of mafia behavior in the Brown-headed Cowbird (Molothrus ater), a widely distributed North American brood parasite. They manipulated ejection of cowbird eggs and cowbird access to predator-proof nests of Prothonotary Warblers to test experimentally for mafia behavior. When cowbird access was allowed, 56% of "ejector" nests were depredated compared with only 6% of "accepter" nests. No nests were destroyed when cowbird access was always denied or when access was denied after they removed cowbird eggs, indicating that cowbirds were responsible. Nonparasitized nests were depredated at an intermediate rate (20%) when cowbirds were allowed access, suggesting that cowbirds may occasionally "farm" hosts to create additional opportunities for parasitism. Cowbirds parasitized most (85%) renests of the hosts whose nests were depredated. Ejector nests produced 60% fewer host offspring than accepter nests because of the predatory behavior attributed to cowbirds. Widespread predatory behaviors in cowbirds could slow the evolution of rejection behaviors and further threaten populations of some of the >100 species of regular cowbird hosts.
Insights into Brown-headed Cowbird behavior by Jeff Hoover
Host responses to brood parasites:

A Warbling Vireo removing the egg of a Brown-headed Cowbird from its nest
(Underwood and Sealy 2006).
Seminar - Cooperative breeding in the tropics by Christie Riehl (seminar begin at 2:53)
Seminar - Cooperation, conflict, and climate change: Pied Babblers and Western Magpies by Amanda Ridley
Cooperative Breeding:
Possible explanations for the evolution of
cooperative breeding:
|
Helpers |
Breeders |
|
|
|
|
|
|
|
|
|
|
|
|
|
A 'test' of these hypotheses:
Gray-crowned Babblers:

Why do helpers 'stay at home' & help?

Cooperative breeding birds in the Israeli desert: Arabian Babblers
Main hypotheses put forward to explain the evolution of delayed independent reproduction (DR) and dispersal.
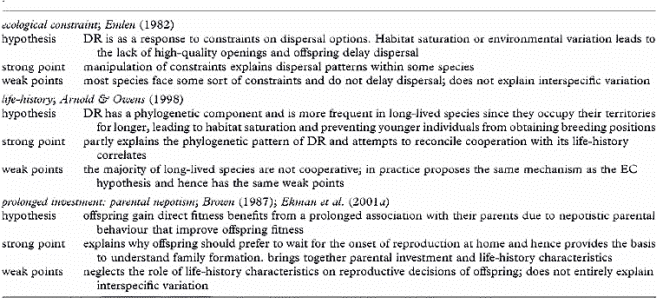
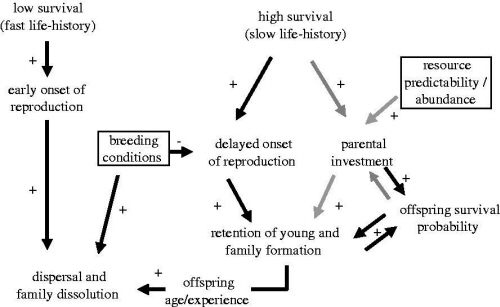
Proposed pathway leading to family formation in birds. The effects of life history and environmental factors on parental decisions are illustrated by the grey arrows, while the effects on offspring decisions are shown by the black arrows. The environmental factors are shown in boxes. The directions of the relationships between traits or factors are illustrated by 'plus' for positive correlations and 'minus' for negative correlations. Species with high survival tend to start breeding later in life and can invest more in offspring. These two factors combined can cause offspring to delay dispersal leading to the formation of families. Environmental factors such as good breeding conditions will promote dispersal. Resource availability allows parents to invest more in offspring without incurring strong costs. However, parents should only prolong investment in offspring if this increases offspring survival prospects substantially without compromising their own survival, and thus there should not be parental investment when offspring survival is too low or too high.
Life history and the evolution of family living in birds -- The reason why some bird species live in family groups is an important question of evolutionary biology that remains unanswered. Families arise when young delay the onset of independent reproduction and remain with their parents beyond independence. Explanations for why individuals forgo independent reproduction have hitherto focused on dispersal constraints, such as the absence of high-quality breeding openings. However, although constraints successfully explain within-population dispersal decisions, they fail as an ultimate explanation for variation in family formation across species. Most family-living species are long-lived and recent life-history studies demonstrate that a delayed onset of reproduction can be adaptive in long-lived species. Hence, delayed dispersal and reproduction might be an adaptive life-history decision rather than 'the best of a bad job.' Covas & Griesser (2007) suggest that longevity favors a delayed onset of reproduction and gives parents the opportunity of a prolonged investment in offspring, an option that is not available for short-lived species. Yet, parents should only prolong their investment in offspring if this increases offspring survival and outweighs the fitness cost that parents incur, which is only possible under ecological conditions, such as a predictable access to resources. Covas & Griesser (2007) therefore proposed that both life-history and ecological factors play a role in determining the evolution of family living across species.
Life-history strategies play a role through favoring a delayed onset of reproduction in long-lived species, i.e. individuals in these species should prefer to breed when they are more experienced and can have access to better territories (or wait for better breeding conditions). Simultaneously, only long-lived species can afford prolonged parental investment. Moreover, any prolonged investment should be guided by changes in survival prospects of both parents and offspring. Parents should only invest in prolonged care to their offspring if this investment substantially improves offspring survival and discontinue investing when this improvement is no longer significant (e.g. when offspring acquire better skills). Also, parents should only invest in offspring beyond independence if they have a predictable access to resources and in the absence of within-family competition for resources. Hence, similarly to the Life History (LH) Hypothesis, Covas & Griesser (2007) suggest that the evolution of family living results from a combination of life-history predisposition and ecological facilitation. However, the processes proposed by Covas & Griesser (2007) differ from those suggested by the LH hypothesis: slow life histories predispose clades to family living, given the intrinsic tendency to delay the onset of independent reproduction and the possibility for parents of a prolonged investment in offspring. In addition, ecological characteristics determine whether or not parents can afford to share resources or provide other forms of protection to their offspring. Finally, within species or populations, the frequency and quality of breeding openings should influence individual decisions on independent breeding, i.e., in agreement with Ecological Constraints hypothesis.
However, the proposed framework does not exclude the possibility that family associations might arise through different pathways. For example, cooperatively breeding Long-tailed Tits (Aegithalos caudatus) are short-lived and individuals always attempt to breed in their first year of life, as predicted by life-history theory. Interestingly, in this species, family cohesion is reached through a different pathway. Owing to high nest predation, population density is low, reducing kin competition and giving offspring the option to delay dispersal and remain associated with their parents throughout their first winter. Then, they disperse and attempt to reproduce independently, but return to the parental territory to help their relatives if their own breeding attempt fails. In this species, family living seems unrelated to life-history characteristics and appears instead linked to environmental factors that allow the family structure to be kept after the breeding season and to their nesting in close spatial association.
Long-tailed Tits

A Superb Starling, a cooperative breeding savanna dweller abundant throughout northeast Africa (Dustin R. Rubenstein photo).
Environmental Variability Drives the Evolution of Cooperative Breeding in Birds -- Many vertebrates breed in cooperative groups in which more than two members provide care for young. Studies of cooperative breeding behavior within species have long highlighted the importance of environmental factors in mediating the paradox of why some such individuals delay independent breeding to help raise the offspring of others. In contrast, studies involving comparisons among species have not shown a similarly clear evolutionary-scale relationship between the interspecific incidence of cooperative breeding and any environmental factors. Rubenstein and Lovette 2007) used a phylogenetically controlled comparative analysis of a complete, socially diverse group of birds — 45 species of African starlings — to show that cooperative breeding is positively associated with living in semiarid savanna habitats and with temporal variability in rainfall. Savanna habitats are not only highly seasonal, but also temporally variable and unpredictable, and this temporal variability directly influences individual reproductive decisions in starlings and helps explain interspecific patterns of sociality. Cooperative breeding is likely to be adaptive in temporally variable environments because it allows for both reproduction in harsh years and sustained breeding during benign years. This “temporal variability” hypothesis might help explain the phylogenetic and geographic concentrations of cooperatively breeding vertebrates in savanna-like habitats and other temporally variable environments worldwide.
Literature cited:
Andersson, M. and M. Åhlund. 2000. Host-parasite relatedness shown by protein fingerprinting in a brood parasitic bird. Proc. Natl. Acad. Sci. 97: 13188-13193.
Andersson, M. and L. W. Simmons. 2006. Sexual selection and mate choice. Trends in Ecology and Evolution 21: 296-302.
Arnold, K.E. and I. P. Owens. 1998. Co-operative breeding in birds: a comparative test of the life-history hypothesis. Proceedings of the Royal Society B 265: 739-745.
Arnold, K. E. and I. P. F. Owens. 2002. Extra-pair paternity and egg dumping in birds: Life history, parental care, and the risk of retaliation. Proceedings of the Royal Society B 269: 1263–1269.
Barske, J., B. A. Schlinger, M. Wikelski, and L. Fusani. 2011. Female choice for male motor skills. Proceedings of the Royal Society B 278: 3523-3528.
Beecher, M. and I. Beecher. 1979. Sociobiology of Bank Swallows: reproductive strategy of the male. Science 205:1282-1285.
Bertrand, S., B. Faivre, and G. Sorci. 2006. Do carotenoid-based sexual traits signal the availability of non-pigmentary antioxidents? Journal of Experimental Biology 209: 4414-4419.
Brouwer, L. and S. C. Griffith. 2019. Extra-pair paternity in birds. Molecular Ecology 28:4864–4882.
Brown, J. L. 1987. Helping and communal breeding in birds. Princeton University Press, Princeton, NJ.
Brown, J.L. and E.R. Brown. 1981. Extended family system in a communal bird. Science 211:959-960.
Chen, Min, Guopan Li, Jinlong Liu, and Shaobin Li. 2021. Large brain size is associated with low extra‐pair paternity across bird species. Ecology and Evolution 11: 13601-13608.
Chen et al. 2023. Divorce rate in monogamous birds increases with male promiscuity and migration distance. Proceedings of the Royal Society B 290: 20230450.
Covas, R. and M. Griesser. 2007. Life history and the evolution of family living in birds. Proceedings of the Royal Society B 274: 1349-1357.
Ekman, J., V. Baglione, S. Eggers, & M. Griesser. 2001. Delayed dispersal: living under the reign of nepotistic parents. Auk 118: 1-10.
Emlen, S.T. 1982. The evolution of helping. I. An ecological constraints model. American Naturalist 119: 29-39.
Emlen, S.T., Peter H. Wrege, and Michael S. Webster. 1999. Cuckoldry as a cost of polyandry in the sex-role-reversed wattled jacana, Jacana jacana. Proceedings of the Royal Society B 265:2359-2364.
Ericson et al. 2020. Parallel Evolution of Bower-Building Behavior in Two Groups of Bowerbirds Suggested by Phylogenomics. Systematic Biology 69: 820–829.
Forstmeier, W., K. Martin, E. Bolund, H. Schielzeth, and B. Kempenaers. 2011. Female extrapair mating behavior can evolve via indirect selection on males. Proceedings of the National Academy of Sciences 108: 10608-10613.
Fresneau et al. 2024. The evolution of sex roles: The importance of ecology and social environment. Proceedings of the National Academy of Sciences 121: e2321294121.
Gowaty, P. A. 1996. Battles of the sexes and origins of monogamy. Pages 21–52 in Partnerships in Birds: The Study of Monogamy (J. M. Black, Ed.). Oxford University Press, Oxford.
Gowaty, P. A. and W. C. Bridges. 1991. Nestbox availability affects extra-pair fertilizations and conspecific nest parasitism in Eastern Bluebirds, Sialia sialis. Animal Behaviour 41: 661–675.
Griffith, S. C., I. P. F. Owens, and K. A. Thuman. 2002. Extra-pair paternity in birds: A review of interspecific variation and adaptive function. Molecular Ecology 11: 2195–2212.
Hartley, R. C. and M. W. Kennedy. 2004. Are carotenoids a red herring in sexual display? Trends Ecol. Evol. 19: 353 -354.
Hoover, J, P. and S. K. Robinson. 2007. Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proceedings of the National Academy of Science 104: 4479-4483.
Jiguet, F. and V. Bretagnolle. 2006. Manipulating lek size and composition using decoys. American Naturalist 168:758-768.
Johnsen, A., V. Andersen, C. Sunding, & J.T. Lifjeld. 2000. Female bluethroats enhance offspring immunocompetence through extra-pair copulations. Nature 406: 296-299.
Jukema, J. and T. Piersma. 2006. Permanent female mimics in a lekking shorebird. Biology Letters 2: 161-164.
Kempenaers, B. 2022. Mating systems in birds. Current Biology 32: R1042–R1172.
Kilner, R.M., J. R. Madden, and M. E. Hauber 2004. Brood parasitic cowbird nestlings use host young to procure resources. Science 305: 877-879.
Lahti, D.C. 2005. Evolution of bird eggs in the absence of cuckoo parasitism. Proceedings of the National Academy of Sciences USA. 102:18057–18062.
Lahti, D. C. 2006.Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 60: 157-168.
Leisler, B., H. Winkler, and M. Wink. 2002. Evolution of breeding systems in Acrocephaline warblers. Auk 119:379-390.
Lyon, B. E. and J. M. Eadie. 2000. Family matters: Kin selection and the evolution of conspecific brood parasitism. Proc. Natl. Acad. Sci. 97:12942-12944.
Lyu et al. 2025. A synthesized framework for the evolutionary origins of avian obligate brood parasitism. Science Advances 11: eadx8063.
Mikula et al. 2022. A global analysis of aerial displays in passerines revealed an effect of habitat, mating system and migratory traits. Proceedings of the Royal Society B 289: 20220370.
Neodorf, D. L. H. 2004. Extrapair paternity in birds: understanding variation among species. Auk 121: 302-307.
Payne, R. B. 1998. Brood parasitism in birds: strangers in the nest. BioScience 48:377-386.
Peer, B. D. and S. G. Sealy. 2004. Correlates of egg rejection in hosts of the Brown-headed Cowbird. Condor 106:580-599.
Petrie, M., C. Doums, and A. P. Møller. 1998. The degree of extra-pair paternity increases with genetic variability: Proceedings of the National Academy of Sciences USA 95: 9390–9395.
Petrie, M., and B. Kempenaers. 1998. Extra-pair paternity in birds: Explaining variation between species and populations. Trends in Ecology and Evolution 13: 52–58.
Pietz, Pamela J., and Diane A. Granfors. 1998. A miniature camera system for studies of grassland passerine nests. U.S. Geological Survey. Biological Information and Technology Notes No. 98-011. Jamestown, ND: Northern Prairie Wildlife Research Center Home Page. http://www.npwrc.usgs.gov/resource/1999/nestcam/nestcam.htm
Poesel, A., H. P. Kunc, K. Foerster, A. Johnsen and B. Kempenaers. 2006. Early birds are sexy: male age, dawn song and extrapair paternity in Blue Tits, Cyanistes (formerly Parus) caeruleus. Animal Behaviour 72: 531-538.
Sly, N. D., et al. 2022. Molecular parallelism in signaling function across different sexually selected ornaments in a warbler. Proceedings of the National Academy of Sciences 119: e2120482119.
Spottiswoode, C. N., and J. Koorevaar. 2012. A stab in the dark: chick killing by brood parasitic honeyguides. Biology Letters 8: 241-244.
Stutchbury, B. J. and E. S. Morton. 1995. The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour 132: 675–690.
Trainer, J.M., D. B. McDonald, and W. A. Learn. 2002. The development of coordinated singing in cooperatively displaying Long-tailed Manakins. Behavioral Ecology 13:65-69.
Underwood, T. J. and S. G. Sealy. 2006. Grasp-ejection in two small ejecters of cowbird eggs: a test of bill-size constraints and the evolutionary equilibrium hypothesis. Animal Behaviour 71: 409-416.
Valcu et al. 2021. The macroecology of extra-pair paternity in birds. Molecular Ecology 30: 4884-4898.
Westneat, D. F. and P. W. Sherman. 1997. Density and extra-pair fertilizations in birds: A comparative analysis. Behavioral Ecology and Sociobiology 41:205–215.
Winfree, R. 1999. Cuckoos, cowbirds and the persistence of brood parasitism. Trends in Ecology and Evolution 14: 338-343.
Useful links: