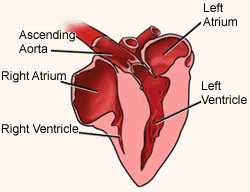
Source: chickscope.beckman.uiuc.edu/explore/ embryology/day02/comparative.html |
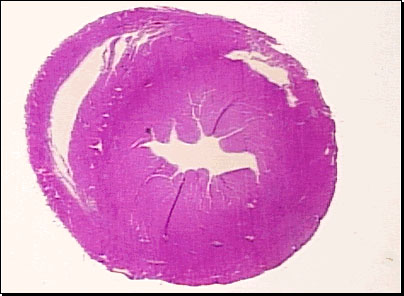
Cross-section through the ventricles of a chicken heart (Source: trc.ucdavis.edu/mjguinan/apc100/modules/ Circulatory/heart/chambers1/chambers.html) |
| BIO 554/754
Ornithology Avian Circulatory System |
Birds have very efficient cardiovascular systems that permit them to meet the metabolic demands of flight (and running, swimming, or diving). The cardiovascular system not only delivers oxygen to body cells (and removes metabolic wastes) but also plays an important role in maintaining a bird's body temperature.The avian circulatory system consists of a heart plus vessels that transport:

Source: chickscope.beckman.uiuc.edu/explore/ embryology/day02/comparative.html |

Cross-section through the ventricles of a chicken heart (Source: trc.ucdavis.edu/mjguinan/apc100/modules/ Circulatory/heart/chambers1/chambers.html) |
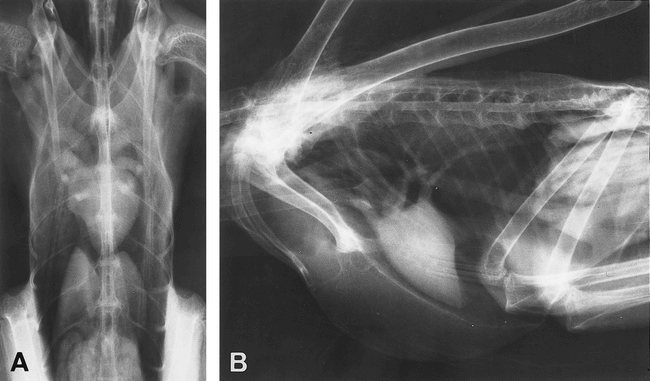
Dorsoventral (A) and lateral (B) thoracic radiographs
from a grey heron, showing the normal avian cardiac silhouettes,
which are located nearly along the longitudinal axis
of the body (Machida and Aohagi 2001).

Heart of a Domestic Chicken. RA, right atrium; RV, right ventricle, LA, left atrium; LV, left ventricle; RAVV, right atrioventricular valve; LAVV, left atrioventricular valve;
IVS, interventricular septum; IAS, interatrial septum; SVC, superior vena cava.
The left atrioventricular valve of birds has three cusps whereas the right AV valve is a single section of myocardium
(Figure modified from Lu et al. 1993).
Birds tend to have larger hearts than mammals (relative to body size and mass). The relatively large hearts of birds may be necessary to meet the high metabolic demands of flight. Among birds, smaller birds have relatively larger hearts (again relative to body mass) than larger birds. Hummingbirds have the largest hearts (relative to body mass) of all birds, probably because hovering takes so much energy.
 |
 |
 |
Avian hearts also tend to pump more blood per unit time than
mammalian hearts. In other words, cardiac output (amount of blood pumped
per minute) for birds is typically greater than that for mammals of the
same body mass. Cardiac output is influenced by both heart rate (beats
per minute) and stroke volume (blood pumped with each beat). 'Active' birds
increase cardiac output primarily by increasing heart rate. In
a pigeon, for example (Butler et al. 1977):
|
|
|
|
|
| Heart rate |
|
|
|
| Stroke volume |
|
|
|
| Cardiac output |
|
|
|
| Oxygen consumed |
|
|
|
In general, bird hearts 'beat' at somewhat lower rates than mammals
of the same size but pump more blood per 'beat.' Among birds, heart rate
varies with size:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Source: Welty & Baptista. 1988. The Life of Birds. Saunders College Publishing, New York.
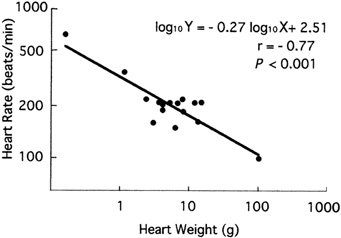
Relationship between heart weight and heart rate at rest
given on a
bilogarithmic scale. The mean value for a given species
is plotted
in this chart (Machida and Aohagi 2001).
Blood pumped by the avian heart enters the blood vessels. The main types are:
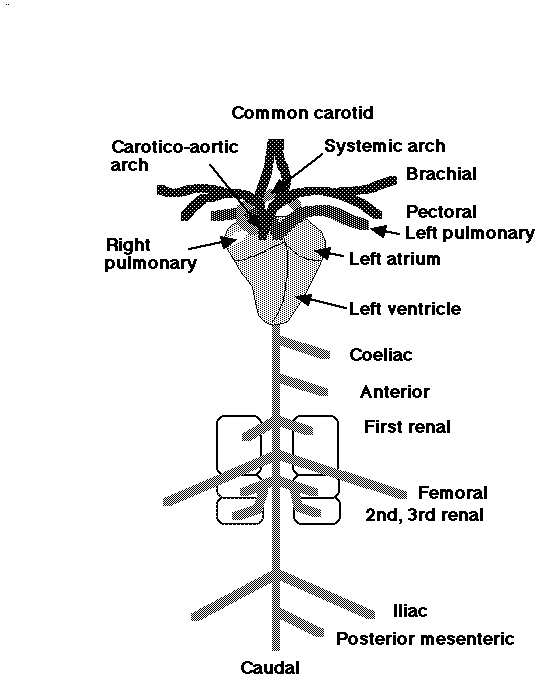
Some of the major arteries in the avian circulatory system:
| Carotids deliver blood to the head (& brain).
Brachials take blood to the wings. Pectorals deliver blood to the flight muscles (pectoralis). The systemic arch is also called the aorta & delivers blood to all areas of the body except the lungs. The pulmonary arteries deliver blood to the lungs. The celiac (or coeliac) is the first major branch of the descending aorta & delivers blood to organs & tissues in the upper abdominal area. Renal arteries deliver blood to the kidneys. Femorals deliver blood to the legs & the caudal artery takes blood to the tail. The posterior mesenteric delivers blood to many organs & tissues in the lower abdominal area. |
Source: http://numbat.murdoch.edu.au/Anatomy/avian/avian3.html |
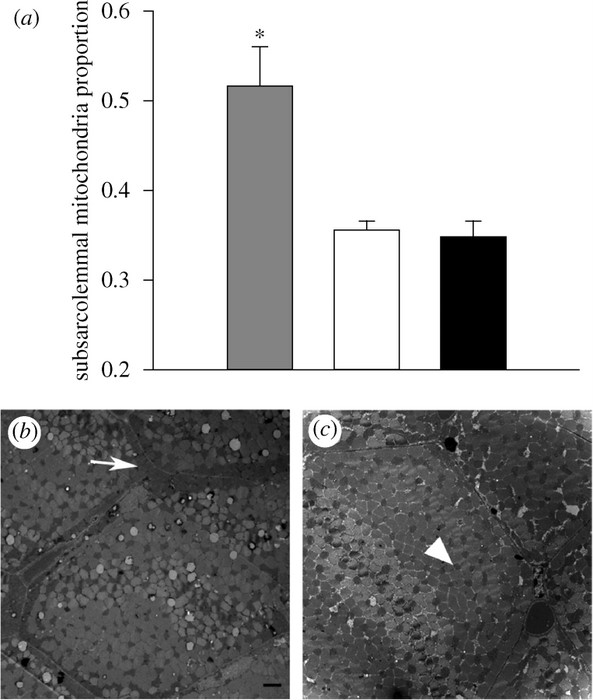
Mitochondria are redistributed towards the cell membrane in the muscle fibers of Bar-headed Geese. (a) The proportion of mitochondria that were subsarcolemmal was higher in Bar-headed Geese than in low altitude species. Grey bar, Bar-headed Goose; unfilled bar, Barnacle Goose; black bar, Pink-footed Foose. (b,c) Representative transmission electron micrographs of muscle fibers from (b) Bar-headed Geese and (c) Barnacle Geese. Scale bar, 2 µm. Arrow, subsarcolemmal mitochondria; arrowhead, intermyofibrillar mitochondrion.
Adaptation for high-altitude flight -- Bar-headed Geese (Anser indicus) migrate over the Himalayas at up to 9000 m elevation, but it is unclear how they sustain the high metabolic rates needed for flight in the severe hypoxia at these altitudes. To better understand the basis for this physiological feat, Scott et al. (2009) compared the flight muscle of Bar-headed Geese to that of low altitude birds (Barnacle Geese, Pink-footed Geese, Greylag Geese, and Mallard ducks). Bar-headed Geese had more capillaries per muscle fiber than expected, and higher capillary densities and more homogeneous capillary spacing. Their mitochondria were also redistributed towards the sarcolemma (cell membrane) and adjacent to capillaries. These alterations should improve O2 diffusion capacity from the blood and reduce intracellular O2 diffusion distances, respectively. Bar-headed Geese have, therefore, evolved for exercise in hypoxia by enhancing the O2 supply to flight muscle.

Bar-headed Geese
BBC Worldwide - Bar-headed Geese
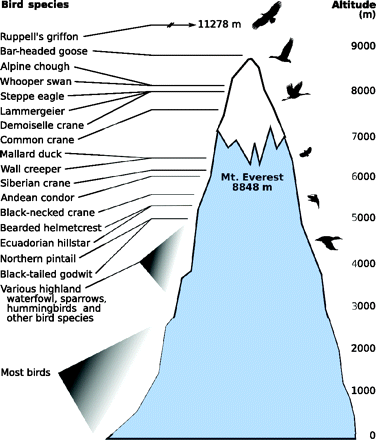
Most birds live and fly at relatively low altitudes, but some species live, migrate,
or are occasionally found at higher altitudes (Source: Scott 2011).
AOS21-(430807) Evolution of blood-oxygen carrying capacity in hummingbirds from American Ornithological Society on Vimeo.
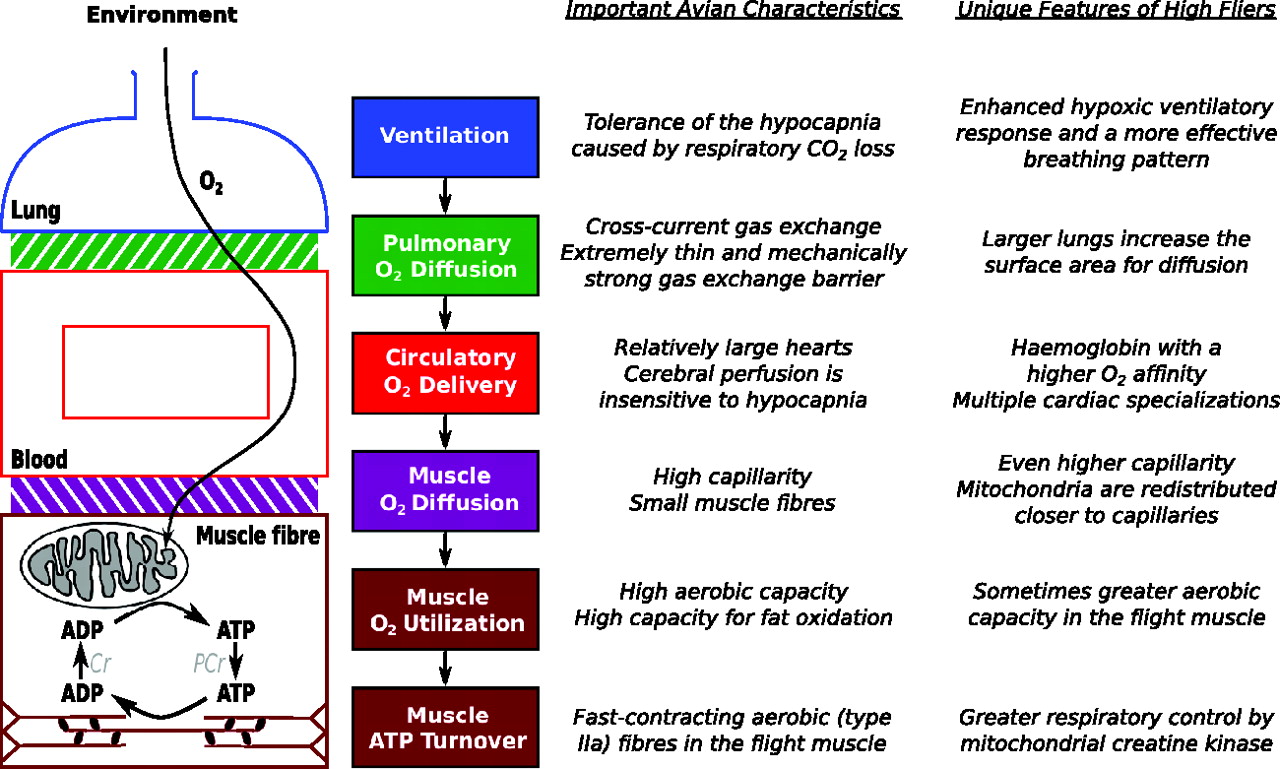
The transport of O2 occurs along several steps of a cascading physiological pathway from atmospheric air to the mitochondria in tissue cells (e.g. muscle fibers). The effectiveness of this pathway at transporting O2 during hypoxia is imperative for flight at high altitudes, which depends upon several distinctive characteristics of birds in general and many unique features that have evolved in high flyers. The properties of O2 utilization and ATP turnover in the flight muscle are also important to consider in high fliers (Source: Scott 2011),
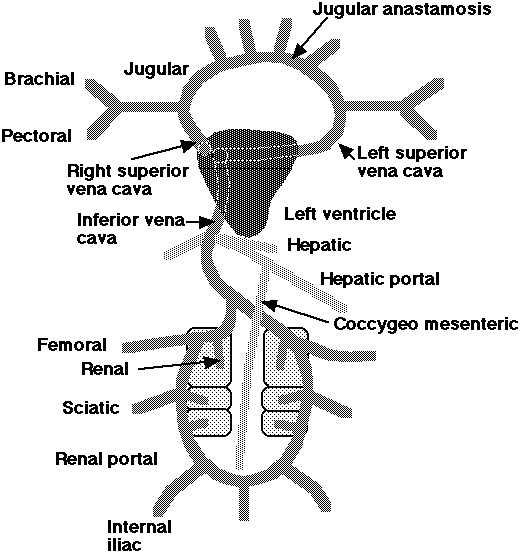
Some major veins in the avian circulatory system:
|
The jugular anastomosis allows blood to flow from right to left side when the birds head is turned & one of the jugulars constricted. The jugular veins drain the head and neck. The brachial veins drain the wings. The pectoral veins drain the pectoral muscles and anterior thorax. The superior vena cavae (or precavae) drain the anterior regions of the body. The inferior vena cava (or postcava) drains the posterior portion of the body. The hepatic vein drains the liver. The hepatic portal vein drains the digestive system. The coccygeomesenteric vein drains the posterior digestive system & empties in the hepatic portal vein. The femoral veins drain the legs. The sciatic veins drain the hip or thigh regions. The renal & renal portal veins drain the kidneys. |
The heart pumps & the vessels carry, of course, blood. Avian blood:
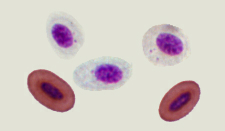 antibodies, & electrolytes.
antibodies, & electrolytes.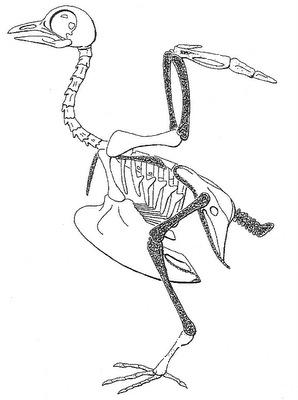 |
Skeleton of a Rock Pigeon (Columba livia) showing the bones (shaded) that contain red-blood-cell-producing marrow, including the radius and ulna of the wing, femur and tibiotarsus of the leg, furcula and scapula of the pectoral girdle, pubis of the pelvic girdle, and caudal vertebrae. Most other bones (except for very small ones) are pneumatized (Schepelmann 1990). |
Differences in the red blood cells of birds and mammals -- Mammals, which had developed an aerobic metabolism, emerged in the Triassic, when the oxygen content in the atmosphere was by approximately 50% lower than current levels and even lower than in the Jurassic period (when birds evolved). Under these conditions, natural selection favored the loss of nuclei in the red blood cells of mammals (making the cells smaller and allowing capilaries to become even smaller in diameter) and change to a biconcave shape (increasing the amount of surface area and enhancing diffusion into and out of the red blood cells). Birds, with their efficient respiratory system, evolved during the Jurassic when the oxygen content in the Earth atmosphere approached the present level, so there was no selective pressure to eliminate nuclei from their red blood cells or change in shape (Gavrilov 2013).
|
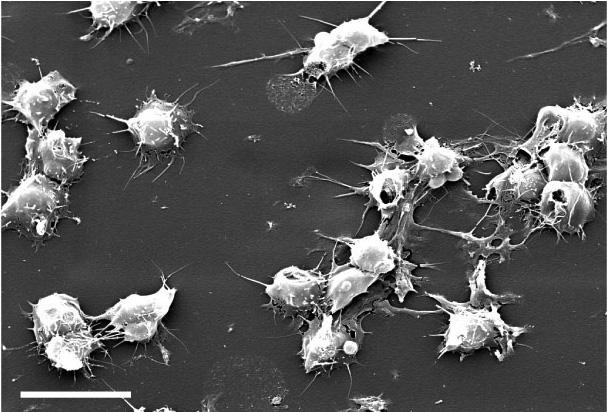
Scanning electron microscope view of bird thrombocytes adhering to a collagen-lined plate (exposure to collagen causes bird thrombocytes, and mammalian platelets, to release chemicals that make them 'sticky'; the chemicals released by mammalian platelets are different from those released by bird thrombocytes and make platelets 'stickier' than thrombocytes). Avian thrombocytes are larger than
mammalian platelets, have a nucleus, and, unlike mammalian platelets, do not form 3-dimensional aggregates. (Credit: Penn Medicine)
Can birds have heart attacks and strokes? -- Mammalian platelets are small, anuclear circulating cells that form tightly adherent (i.e., 'sticky') thrombi (clots or 'plugs') to prevent blood loss after vessel injury. Platelet thrombi that form in the coronary and carotid arteries of humans can also cause common vascular diseases such as myocardial infarction ('heart attacks') and stroke and are the target of drugs used to treat these diseases. Birds have high-pressure cardiovascular systems like mammals, but have nucleated thrombocytes in their blood rather than platelets. Schmaier et al. (2011) found that avian thrombocytes respond to many of the same activating stimuli as mammalian platelets (and so help stop blood loss from damaged vessels) but, unlike mammalian platelets, cannot form tighly adherent thrombi in arteries. Avian thrombocytes are larger than mammalian platelets and are less 'sticky' (because they release different chemicals) than mammalian platelets when exposed to collagen (connective tissue to which thrombocytes and platelets are exposed when there's a break in a blood vessel). When carotid arteries of mice are damaged, platelets form thrombi that can block blood flow (check this video showing the response of human platelets when exposed to a plate covered with collagen); similar damage to the carotid arteries of Budgerigars (similar in size and speed and pressure of blood flow to the carotid arteries of mice) did not cause the formation of thrombi (check this video showing the response of chicken thrombocytes when exposed to a plate covered with collagen). These results indicate that mammalian platelets, in contrast to avian thrombocytes, will form thrombi even in arteries where blood flow is rapid and under high pressure, an essential element in human cardiovascular diseases.
Link:
Heart disease linked to evolutionary changes that may have protected early mammals from trauma
Brain size & immunity -- Parasitism can negatively
affect learning and cognition. Greater susceptibility to parasitism by
males may impair cognitive ability, and greater male investment in immunity
could compensate for greater susceptibility, in particular when males have
a relatively large brain. Why might males be more susceptible to parasites?
These findings support the hypothesis that sex differences in brain function have evolved as a consequence of differences in susceptibility to parasitism. Different components of the immune system (bursa and spleen) may have evolved to mitigate the negative impact of parasites on brain function. |
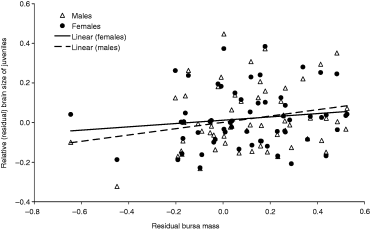
Covariation between relative brain mass of juvenile birds and relative mass of bursa of Fabricius in different bird species. Relative mass was calculated as residuals from a phylogenetically corrected regression of log10-transformed organ mass on log10-transformed body mass. The lines are the linear regression lines for males and females, respectively (From: Møller et al. 2004). |
B-lymphocytes, the cells that produce antibodies, are initially produced
in the embryonic liver, yolk sac and bone marrow, then move through the
blood to the bursa of Fabricius (BF).
| Birds have a bursa of Fabricius (BF), which is an outpocketing
of cloaca. Within the BF, B-lymphocytes mature then migrate to other
body tissues. The bursa is a blind sac that extends from the dorsal side
of the cloaca, the common portal of the reproductive, urinary, and digestive
systems. Within the bursas of young birds are extensive leaf-like folds
composed of simple, columnar epidermis and a loose connective tissue with
lots of blood vessels and lymph nodules. Atrophy of the BF typically occurs
around the time of sexual maturation.
(Source: http://www.upei.ca/~morph/webct/Modules/Lymphoid/bursa.html) |
In the BF, the B-cells mature and become functional and then move to the blood, spleen, cecal tonsils, bone marrow, Harderian gland (Hg in diagram below), and thymus.
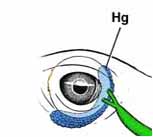
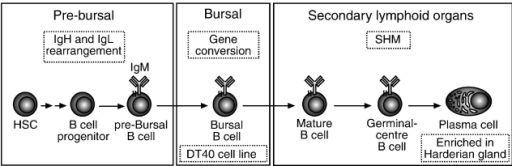
In birds, most of the Ig diversification occurs by gene conversion in the bursa of Fabricius.
However, further Ig diversification is achieved by somatic hypermutation in secondary lymphoid organs (From: Kohonen et al. 2007).
B-lymphocytes produce three classes of antibodies after exposure to a disease organism: IgM, IgY (equivalent to mammalian IgG), and IgA. Ig M appears after 4-5 days following exposure to a disease organism and then disappears by 10-12 days. IgY is detected after 5 days following exposure, peaks at 3 to 3 1/2 weeks, and then slowly decreases. Ig A appears after 5 days following exposure. This antibody is found primarily in the mucus secretions of the eyes, gut, and respiratory tract and provides "local" protection to these tissues.
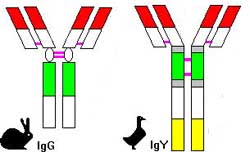
From: Szabo et al. 1998.
Antibodies do not have the capability to kill viruses or bacteria directly. Antibodies (especially IgY) perform their function by attaching to disease organisms (like bacteria) and blocking their receptors. The disease organisms are then prevented from attaching to their target cells. The attached antibodies can also facilitate the destruction of pathogens by phagocytes.

IgY Ab = IgY antibody
Source: http://www.genwaybio.com/technology.htm
T-lymphocytes begin as the same stem cells as the B-cells, but are programmed in the thymus rather than the BF. The T-lymphocytes include a more heterogeneous population than the B-cells. Some T-cells act by producing lymphokines (over 90 different ones have been identified); others directly destroy disease organisms. Some T-cells act to enhance the response of B-cells, macrophages, or other T-cells (helpers); others inhibit the activity of these cells (suppressors).
This video describes B cell development in the chicken. B cells produce antibodies that bind to infectious organisms (viruses, bacteria, and parasites) and play a vital role for the immune system to protect chickens, as well as us, from infectious disease.
| How Did the Peacock Get His Tail? -- It's a question that has puzzled zoologists for more than a century. Charles Darwin first noted that the choosy peahen plays a crucial role in the evolution of this extravagant sexual display. "We may conclude that…those males which are best able by their various charms to please or excite the female, are under ordinary circumstances accepted. If this be admitted, there is not much difficulty in understanding how male birds have gradually acquired their ornamental characters," Darwin wrote. Moller and Petrie (2002) now suggest that plumage may specifically convey the strength of a male's immune system and his desirability as a mate. Hamilton and Zuk (1982) first suggested that 'showy' males were signaling to females that they were, if not parasite-free, then parasite "lite." But, there has been little evidence to support this hypothesis. Moller believes it is because people have been looking at the wrong parasites. "If you look at our own species, we are attacked by hundreds of different species of parasites," said Moller. "So if you wanted to study our parasite burden, you'd have to identify all the parasites, from tapeworms to head lice, see how abundant they are and how they affect us. It would be practically impossible, so we decided to focus on the immune system." Moller and Petrie took blood samples from male Blue Peafowl (Pavo cristatus) and recorded the numbers of B- and T-cells, and also measured the peacocks' tails and counted the number of eye spots. They discovered that the condition and length of the peacock's tail was related to the production of B-cells, and the size of the eye spots to T-cell production. "Our main finding is that females are looking at different aspects of a male's immune competence," said Moller. Males, in effect, are walking billboards advertising their health and status. And these things matter. Previous research has shown that in chickens and quail, at least, the immune system is under genetic control so offspring will inherit their parents' ability to fight parasites. Thus, it pays for females to be choosy because their chicks, in turn, will survive better and mate with other, equally picky females. -- Sanjida O'Connell, The Independent (London), September 9, 2002 |  |
Immunosenescence in some immune components of free-living Tree Swallows -- A wide diversity of free-living organisms show increases in mortality rates and/or decreases in reproductive success with advancing age. However, the physiological mechanisms underlying these demographic patterns of senescence are poorly understood. Immunosenescence, the age-related deterioration of immune function, is well documented in humans and in laboratory models, and often leads to increased morbidity and mortality due to disease. However, little is known about immunosenescence in free-living organisms. Palacios et al. (2007) studied immunosenescence in a free-living population of Tree Swallows (Tachycineta bicolor), assessing three components of the immune system and using both in vivo and in vitro immunological tests. Immune function in female Tree Swallows showed a complex pattern with age; acquired T-cell mediated immunity declined with age, but neither acquired nor innate humoral immunity did. In vitro lymphocyte proliferation stimulated by T-cell mitogens decreased with age, suggesting that reduced T-cell function might be one mechanism underlying the immunosenescence pattern of in vivo cell-mediated response recently described for this same population. These results provide the most thorough description of immunosenescence patterns and mechanisms in a free-living vertebrate population to date. Future research should focus on the ecological implications of immunosenescence and the potential causes of variation in patterns among species.
The avian cardiovascular system is able to quickly respond to changes in levels of activity (e.g., resting vs. flying) via changes in heart rate, cardiac output, & blood flow (by vasocontriction and vasodilation of vessels).
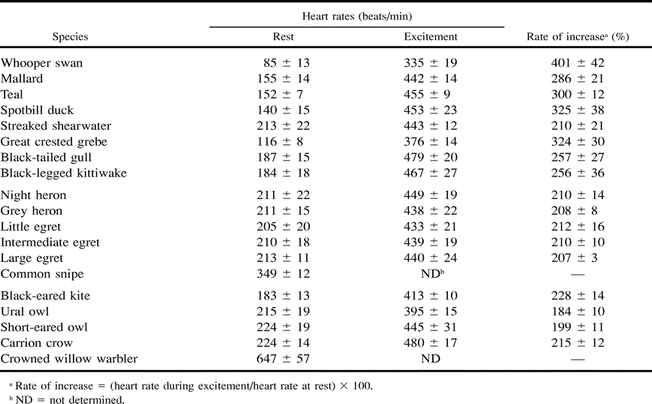
Measurements of resting heart rate were obtained only
after each bird had ceased activity in the dark cage and remained quiet.
The heart rate in an excited state (during excitement)
was measured when the animal became maximally excited because its
movement in the cage was restrained manually (Machida
and Aohagi 2001).
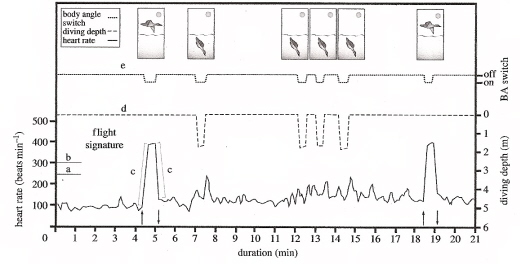
Heart rate, diving depth and body angle of a female Common Eider (Somateria mollissima) during dives and when flying. (a, b) heart rates of 250 and 300 beats per minute, (c) heart rate ascending and descending slopes equal to or above 10 beats per minute per second (absolute values), (d) standard deviation of diving depth up to 0.1 m, and (e) change in body angle. The upward and downward pointing arrows indicate the point of take-off and landing, respectively (From: Pelletier et al. 2007).
Common Eider
(male)
Many birds forage by diving underwater. So, what happens when a bird doesn't have access to air (oxygen)? The quintessential avian diver is the Emperor Penguin, which can attain depths greater than 500 meters while staying submerged for about 12 minutes. In shallower dives, an Emperor Penguin may stay submerged even longer, over 20 minutes. Diving birds, including Emperor Penguins, must 'solve' several problems:
Emperor Penguins
| Diving beyond the limits (Butler 2001) -- Gentoo Penguins around South Georgia feed on krill at depths of 80–90 m. King Penguins feed on myctophid fish at depths of 100–250 m. A large proportion of the dives of both species are longer than their calculated aerobic dive limits. Part of this discrepancy is probably due to inaccuracies in determining rates of oxygen consumption during diving. That this may well be the case is suggested by the fact that the temperature in the abdominal cavity of both Gentoo and King penguins drops during dives and returns to normal when diving behavior ceases. For Gentoo Penguins, abdominal temperature during dives was, on average, 2.4°C lower than when the birds were not diving. The lowest abdominal temperature recorded for each penguin was, on average, 33.6°C for the Gentoos and 29.7°C for the Kings. So if the birds allow temperature to fall in some parts of the body (i.e., they do not increase metabolic rate in an attempt to keep abdominal temperature normal), there will be a saving in energy both in terms of thermoregulatory costs and in terms of reduced metabolic rates in those tissues (i.e, a Q10 effect, with Q10 being the factorial change in the rate of a chemical reaction associated with a 10°C change in temperature). |

Gentoo Penguins
Traces of dive depth (top) and temperature in the abdominal cavity (bottom) during a foraging trip of a Gentoo Penguin (A; mass = 6.6 kg) during 1 day at sea and a King Penguin (B; mass = 11.7 kg) during 5 days at sea. Note change in scale of the depth traces (Butler 2001). |
Gentoo Penguins
Additional physiological responses allow diving birds to make the best use of available oxygen & minimize heat loss in cold water:

Source: http://www.zoo.utoronto.ca/stephenson/Research/Diving.htm
Guillemots
 prevent
the bends. In human divers, increased underwater pressure forces nitrogen
in the air within body cavities to pass into the blood. If divers surface
before the nitrogen is cleared, they can suffer contorted joints, difficult
breathing, and even paralysis. Many whales and seals have blood and muscles
adapted to conserve oxygen; they can also collapse their lungs before diving
to squeeze out air. Penguins don't have it that easy: Their lungs don't
collapse, and the buoyant divers need a good dose of oxygen to swim hard.
To find out how penguins move about at depth, Katsufumi Sato and colleagues
(Sato
et al. 2002) attached data loggers to Adélie and king penguins
off the shores of Antarctica and Crozet Island, about 1000 kilometers away.
The instruments measured the depths, speed, and acceleration and deceleration
effects from wing strokes for more than 650 dives. From these data Sato's
team estimated air volumes in penguin lungs during their descents and ascents.
The dive profiles revealed that the penguins flapped their flippers continuously
on the way down. On return trips, after swimming halfway up, they stopped
and let their natural buoyancy give them a free ascent. But surprisingly,
instead of shooting straight up the penguins veered at an oblique angle,
thus significantly slowing their ascent, the team reports in the May issue
of the Journal of Experimental Biology. This increases the amount of time
the penguins spend in shallow water with little prey, but it could provide
time for nitrogen, under lower pressure, to return to the air inside body
cavities. Those findings intrigue marine biologist Dan Costa of the University
of California, Santa Cruz: They've made careful and insightful measurements
of the fine-scale diving behavior of two penguins, supported with very
sophisticated models of lung volume, and they may be correct. However,
he cautions, there are alternate explanations for why penguins would slow
their ascents, such as looking out for predators. -- Noreen
Parks, Academic Press Daily InScight
prevent
the bends. In human divers, increased underwater pressure forces nitrogen
in the air within body cavities to pass into the blood. If divers surface
before the nitrogen is cleared, they can suffer contorted joints, difficult
breathing, and even paralysis. Many whales and seals have blood and muscles
adapted to conserve oxygen; they can also collapse their lungs before diving
to squeeze out air. Penguins don't have it that easy: Their lungs don't
collapse, and the buoyant divers need a good dose of oxygen to swim hard.
To find out how penguins move about at depth, Katsufumi Sato and colleagues
(Sato
et al. 2002) attached data loggers to Adélie and king penguins
off the shores of Antarctica and Crozet Island, about 1000 kilometers away.
The instruments measured the depths, speed, and acceleration and deceleration
effects from wing strokes for more than 650 dives. From these data Sato's
team estimated air volumes in penguin lungs during their descents and ascents.
The dive profiles revealed that the penguins flapped their flippers continuously
on the way down. On return trips, after swimming halfway up, they stopped
and let their natural buoyancy give them a free ascent. But surprisingly,
instead of shooting straight up the penguins veered at an oblique angle,
thus significantly slowing their ascent, the team reports in the May issue
of the Journal of Experimental Biology. This increases the amount of time
the penguins spend in shallow water with little prey, but it could provide
time for nitrogen, under lower pressure, to return to the air inside body
cavities. Those findings intrigue marine biologist Dan Costa of the University
of California, Santa Cruz: They've made careful and insightful measurements
of the fine-scale diving behavior of two penguins, supported with very
sophisticated models of lung volume, and they may be correct. However,
he cautions, there are alternate explanations for why penguins would slow
their ascents, such as looking out for predators. -- Noreen
Parks, Academic Press Daily InScight
Literature Cited:
Butler, P. J. 2001. Diving beyond the limits. News in Physiological Sciences 16: 222-227.
Butler, P. J., N. H. West, and D. R. Jones. 1977. Respiratory and cardiovascular responses of the pigeon to sustained, level flight in a wind tunnel. Journal of Experimental Biology 71:7-26.
Gavrilov, V. M. 2013. Origin and development of homoiothermy: a case study of avian energetics. Advances in Bioscience and Biotechnology 4:1-17.
Hamilton, W. D. and M. Zuk. 1982 Heritable true fitness and bright birds: a role for parasites? Science 218: 384-387.
Kohonen, P., K.-P. Nera, and O. Lassila. 2007. Avian model for B-Cell immunology - new genomes and phylotranscriptomics. Scandinavian Journal of Immunology 66: 113–121.
Lu, Y., T. N. James, M. Bootsma, and F. Terasaki. 1993. Histological organization of the right and left atrioventricular valves of the chicken heart and their relationship to the atrioventricular Purkinje ring and the middle bundle branch. Anatomical Record 235: 74-86.
Machida, N. and Y. Aohagi. 2001. Electrocardiography, heart rates, and heart weights of free-living birds. Journal of Zoo and Wildlife Medicine 32: 47–54.
Møller, A. P., J. Erritzøe, & L. Z.
Garamszegi. 2004. Covariation between brain size and immunity in birds:
implications for brain size evolution.
Journal of Evolutionary Biology 18: 223-237.
Møller, A.P. and M. Petrie. 2002. Condition dependence, multiple sexual signals, and immunocompetence in peacocks. Behavioral Ecology 13:248–253.
Oglesbee, B. L., R. L. Hamlin, H. Klingaman, J. Cianciola, and S. P. Hartman. 2001. Electrocardiographic Reference Values for Macaws (Ara sp.) and Cockatoos (Cacatua sp.). Journal of Avian Medicine and Surgery 15: 17-22.
Palacios, M. G., J. E. Cunnick, D. W. Winkler, and C. M. Vleck. 2007. Immunosenescence in some but not all immune components in a free-living vertebrate, the Tree Swallow. Proc. Royal Academy of London B, online early.
Pelletier, D., M. Guillemette, J.-M. Grandbois, and P. J. Butler. 2007. It is time to move: linking flight and foraging behaviour in a diving bird. Biology Letters 3: 357-359.
Sato, K., Y. Naito, A. Kato, Y. Niizuma, Y. Watanuki, J. B. Charrassin, C.-A. Bost, Y. Handrich, and Y. Le Maho. 2002. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 205: 1189-1197.
Schepelmann, K. 1990. Erythropoietic bone marrow in the pigeon: development of its distribution and volume during growth and pneumatization of bones. Journal of Morphology 203: 21-34.
Schmaier, A. A., T. J. Stalker, J. L. Runge, D. Lee, C. Nagaswami, P. Mericko, M. Chen, S. Cliche, C. Gariepy, L. F. Brass, D. A. Hammer, J. W. Weisel, K. Rosenthal, and M. L. Kahn. 2011. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood 118: 3661-3669.
Scott, G. R. 2011. Elevated performance: the unique physiology of birds that fly at high altitudes. Journal of Experimental Biology 214: 2455-2462.
Scott, G. R., S. Egginton, J. G. Richards, and W. K. Milsom. 2009. Evolution of muscle phenotype for extreme high altitude flight in the Bar-headed Goose. Proceedings of the Royal Society B: online early.
Szabo, Cs., L. Bardos, S. Losonczy, and K. Karchesz. 1998. Isolation of Antibodies from Chicken and Quail Eggs. Presented at INABIS '98 - 5th Internet World Congress on Biomedical Sciences at McMaster University, Canada, Dec 7-16th. Available at URL http://www.mcmaster.ca/inabis98/immunology/szabo0509/two.html
More lecture notes: