Urinary System, Salt Glands, and Osmoregulation
Osmoregulation refers to the various mechanisms by which birds regulate water and electrolyte levels in their bodies. Body fluids, most importantly extracellular osmolality and blood volume, are regulated within narrow limits. The ‘osmo-‘ in osmoregulation refers to osmosis, the process by which water passes through semipermeable membranes (like cell membranes) in response to differences in solute concentrations. Of course, living cells can only survive if the concentrations of solutes, or electrolytes, remain very similar inside (intracellular fluid) and outside (extracellular fluid) of cells. Insuring that this is the case requires that both water and electrolyte levels in the body be regulated very precisely. In mammals, the kidneys play the critical role in this process. In birds, on the other hand, the kidneys, lower intestine, and, in some species, salt glands all play important roles in osmoregulation.
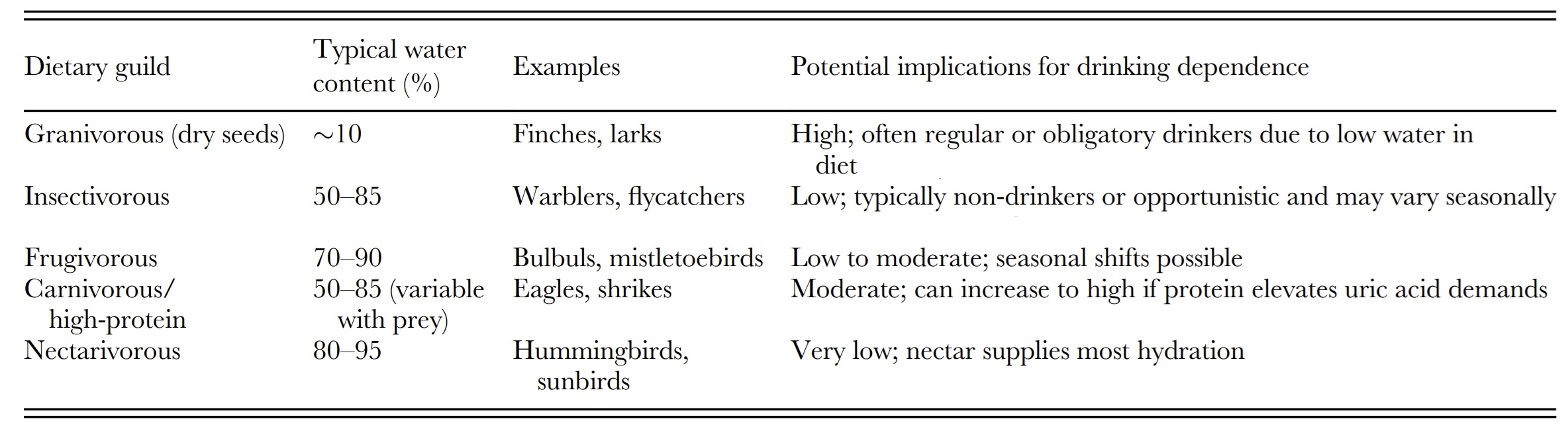
Water balance requires the input matches output. Most birds can obtain water directly by drinking (or, during winter in cold climates, by eating snow - see video below). Birds can also obtain water via the foods they ingest. For example, carnivores ingest animal tissue that is primarily water, frugivores consume fruits containing water, and the nectar consumed by nectarivores is, of course, largely water. In the Sonoran Desert of the southwestern United States and northwestern Mexico, a cactus plant (saguaro, Carnegiea gigantean) appears to be an important source of water for several species of birds (Wolf and de Rio 2003). Even foods that seemingly contain little water can serve as a water source because water is produced as a by-product of cellular metabolism. This metabolic water can be of particular importance to birds in arid environments (Williams 1996). For example, metabolic water represents about 14% of the total water needs of Ostriches in the Namib Desert (Williams et al. 1993). The amount of water formed during fuel oxidation depends on a variety of factors, including metabolic rate and fuel type (carbohydrates, fats, or proteins; Schmidt-Nielsen 1997). Catabolism of fat alone produces about 27 µl of water per kilojoule, whereas catabolizing a mixture of 70% fat and 30% protein yields about 34 µl of water per kilojoule (Klaassen 1996).
Typical water content of diet per dietary guild and the potential implications for drinking dependence in birds (From: Conradie and Freeman 2026).

Flies as a source of water in the Sahara Desert
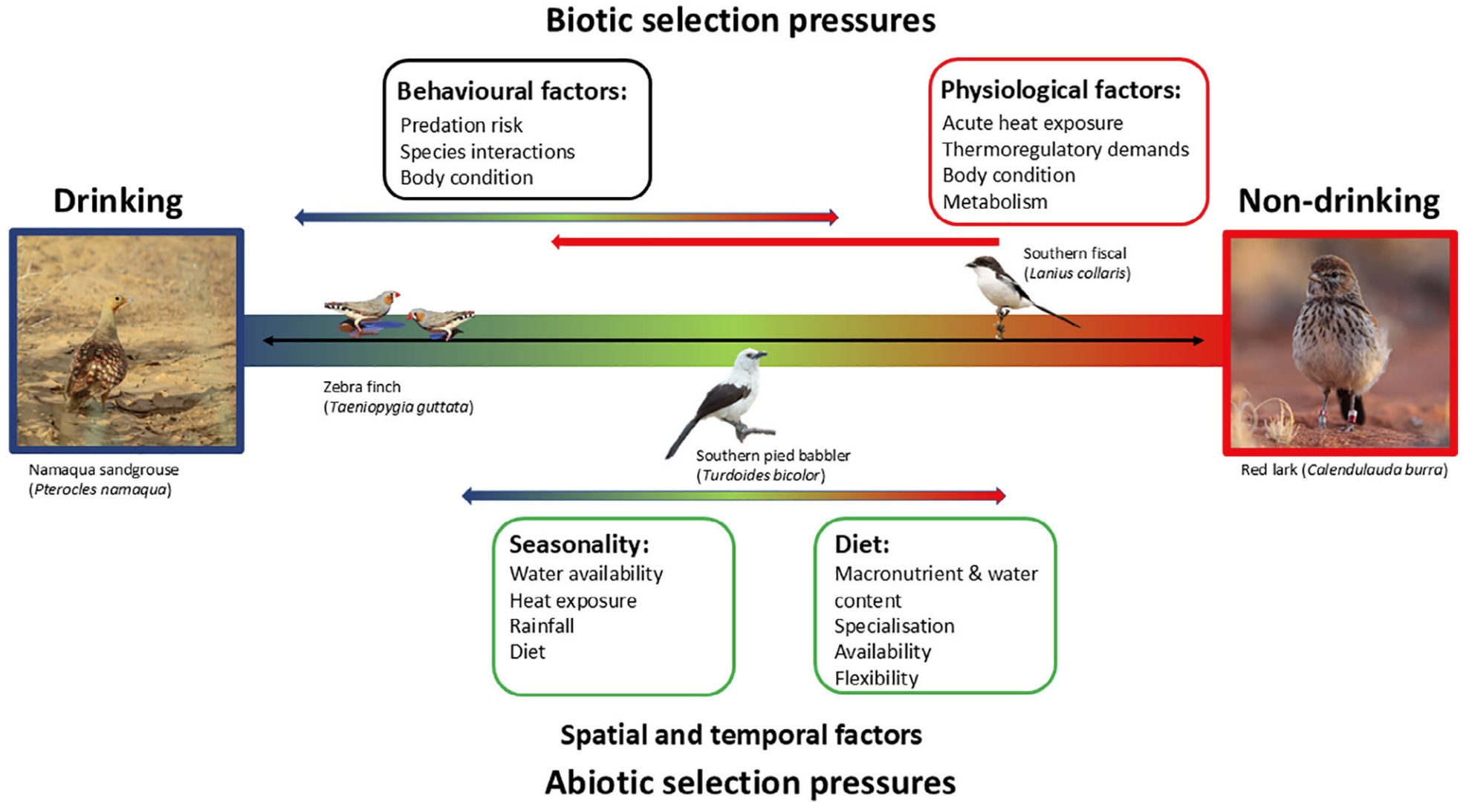
Drinking habits classification in relation to thermal physiology, behavior and the environment. This classification schematic
illustrates
drinking versus non-drinking birds as a continuum with abiotic (i.e., seasonality and diet) and biotic (i.e., behavior and
physiology) pressures
driving species placement between one end and the other rather than being a dichotomous classification (From: Conradie and Freeman 2026).
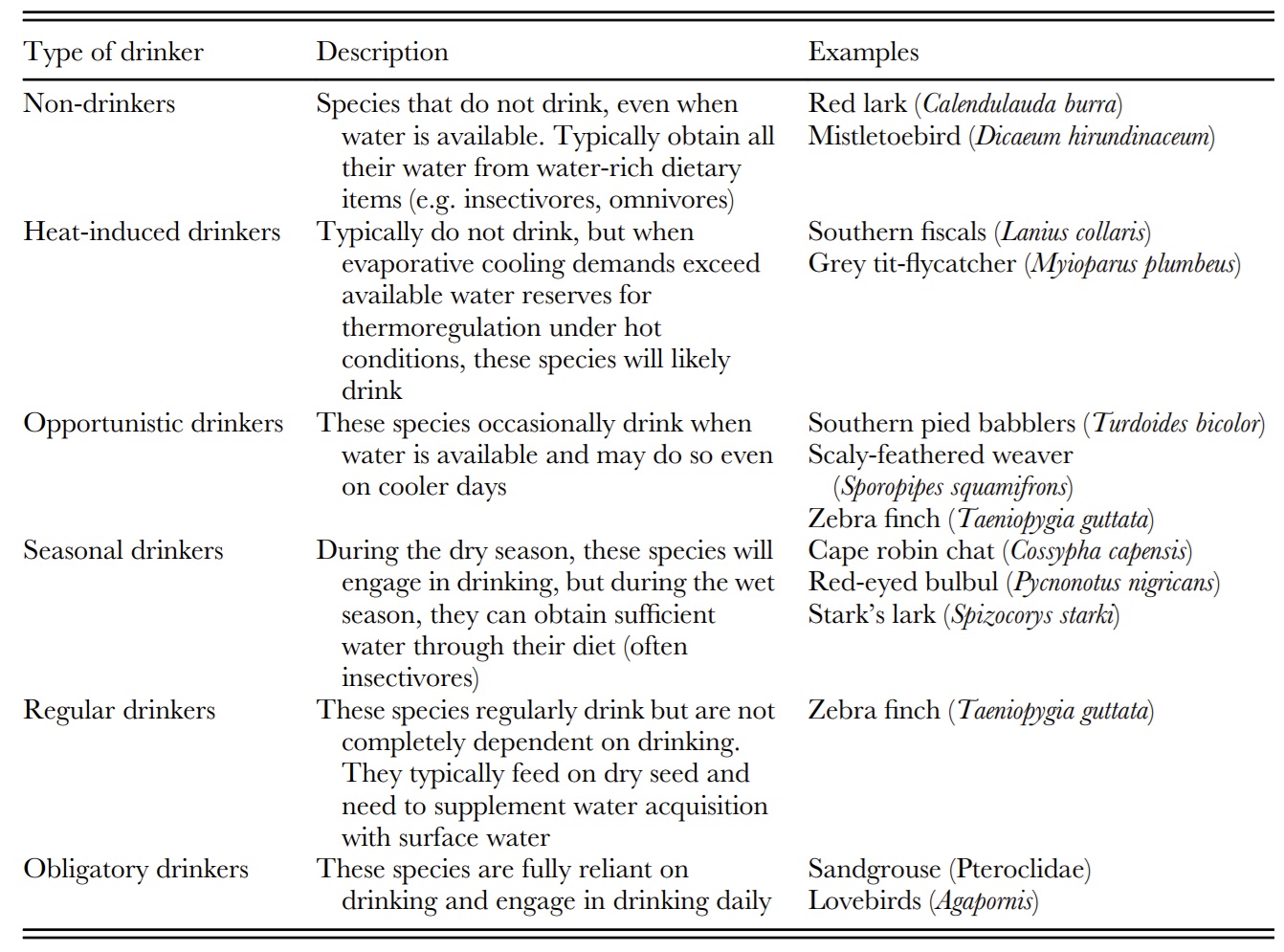
Avian drinking behavior along a gradient. Categories are based on abiotic (e.g. seasonality, temperature, diet) and
biotic (e.g. behavior, physiology) influences. These classifications are not discrete or fixed but exist
along a continuum
from non-drinking to obligate drinking, with species potentially falling into more than one category depending on
environmental and physiological conditions (From: Conradie and Freeman 2026).
How do birds drink?-- Birds generally drink by what's called 'dipping and tipping' (below) or by suction drinking.. Within each of these two main categories, however, there are several kinds of mechanisms. For example, water is taken into the mouth by scooping water with the lower beak in cockatoos (Cacatuinae). Mallards drink using a complex interplay of capillary action and pressure changes in different areas of the mouth. Among the suction drinkers, parakeets ladle water with the tip of the tongue and some parrots drink with a suctioning action. Pigeons and doves, on the other hand, use a "double-suction mechanism" in which capillary action is responsible for bringing water between the slightly gaping tips of the beak and then the tongue acts as a piston to pump the water into the pharyngeal cavity.
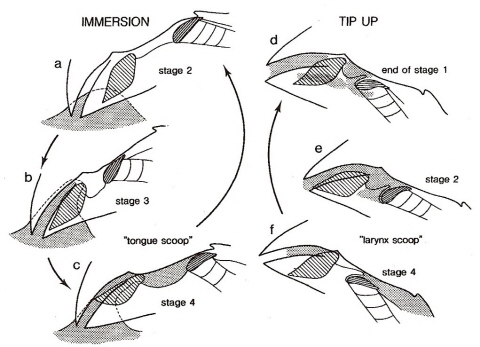
Drinking by a Bengalese Finch. Water runs between the beak tips as a result of adhesion and capillary action.
The bill then tips up and that, along with tongue movements, moves water into the pharynx (Heidweiller and Zweers 1990).
Sandhill Cranes drinking
Mourning Dove drinking
(notice that doves don't have to tip their heads up like most other birds)
Ostrich drinking
Drink safely: Common Swifts (Apus apus) dissipate mechanical energy to decrease flight speed before touch-and-go drinking -- Flight is an efficient way of transport over a unit of distance, but it can be very costly over each unit of time, and reducing flight energy expenditures is a major selective pressure in birds. The Common Swift is one of the most aerial bird species, performing most behaviours in flight: foraging, sleeping, and also drinking by regularly descending to various waterbodies and skimming over the surface. An energy-saving way to perform such touch-and-go drinking would be to strive to conserve mechanical energy, by transforming potential energy to kinetic energy during the gliding descent, touching water at high speed, and regaining height with minimal muscular work. Using 3D optical tracking, Ruaux et al. (2023) recorded 163 swift drinking trajectories, over three waterbodies near Rennes, France. Contrarily to the energy conservation hypothesis, these authors found that swifts approaching a waterbody with a higher mechanical energy (higher height and/or speed 5 s before contact) do not reach water at higher speeds, but do brake, i.e., dissipate mechanical energy to lose both height and speed. Braking seemed to be linked with sharp turns and the use of headwind to some extent, but finer turns and postural adjustments, beyond the resolving power of our tracking data, could also be involved. Ruaux et al. (2023) hypothesize that this surprisingly costly behaviour results from a tradeoff between energy expenditure and safety, because approaching water surface requires fine motor control, and high speed increases the risk of falling into water, which would have serious energetic and survival costs for a swift.
The role of bird kidneys (Figure 1), like the kidneys of other vertebrates is filtration, excretion or secretion, and absorption. They filter water and some substances from blood, such as waste products of metabolism and ions, that are voided in the urine. Kidneys also play an important role in conserving water and reabsorbing needed substances (like glucose).
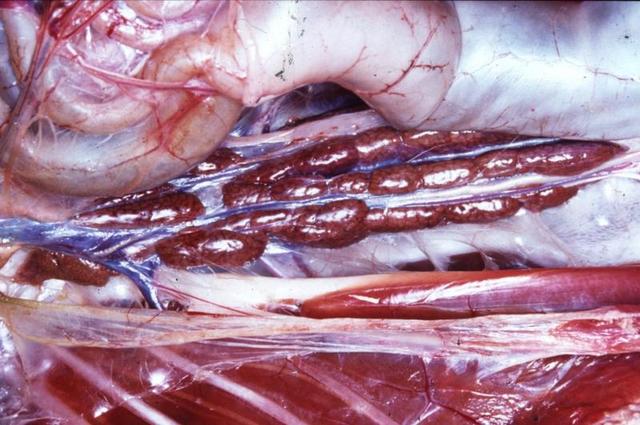
Figure 1. Avian kidneys
http://137.222.110.150/restricted/gallery/album181
The urinary organs of birds consist of paired kidneys and the ureters (Figure 2), which transport urine to the urodeum of the cloaca. Avian kidneys are divided into units called lobules. Each lobule has a
cortex (outer area) and medulla (or medullary cone; Figure 3). In terms of volume, the avian kidney is primarily cortex (71-81%), plus a relatively small medulla (range 5-15%) & blood vessels larger than capillaries (range 10-13%).
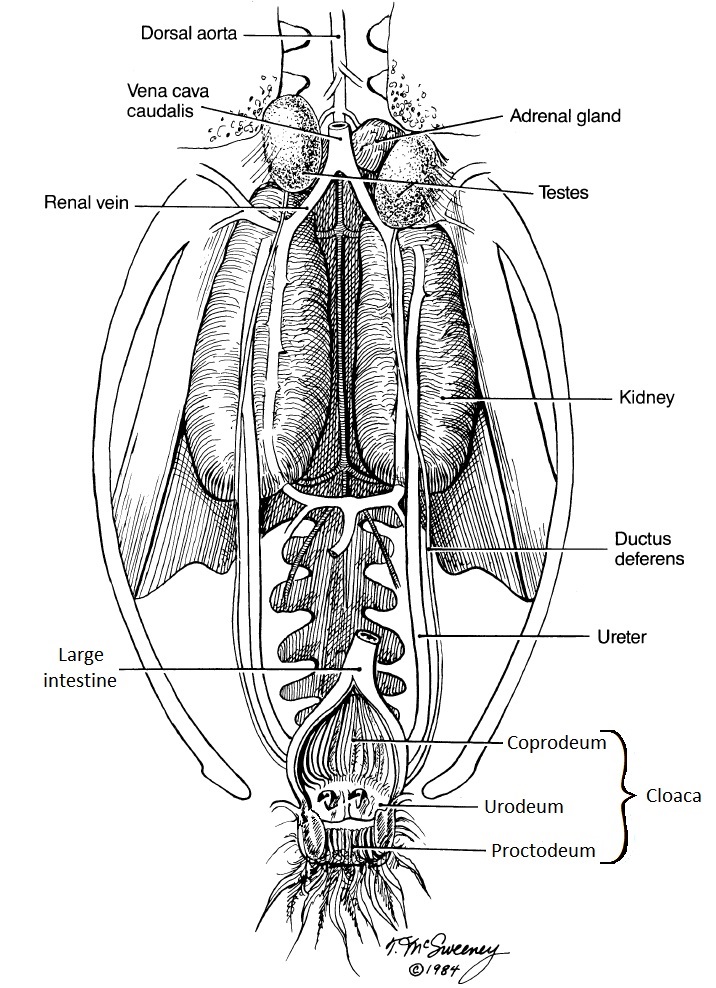
Figure 2. Urine is carried from the avian kidneys to the cloaca (and, specifically, the middle section called the urodeum) by the ureters
(Figure from McSweeney and Stoskopf 1989).
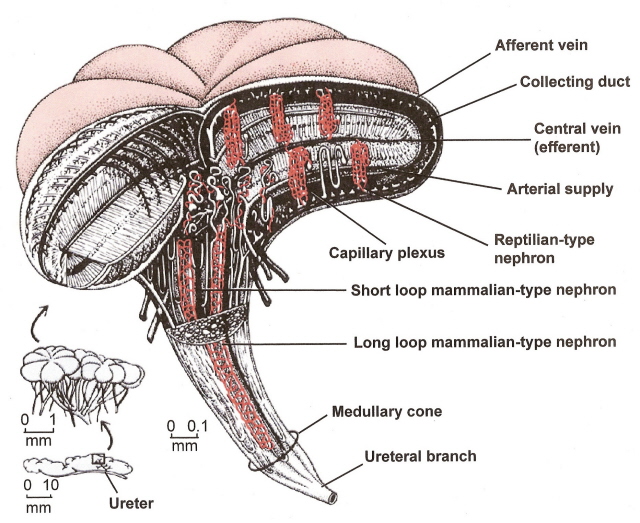
Image from Sherwood et al. (2005).
Figure 3. Lobule of an avian kidney. The medullary cones include the loops of Henle and collecting ducts of nephrons plus a number of capillaries called the vasa recta.
The avian renal medulla is cone shaped because the number of loops of Henle decreases toward the apex of the medullary cones.
The functional unit of the kidney is the nephron. Avian kidneys have two kinds of nephrons. A reptilian-type, with no loops of Henle are located in the cortex, and a mammalian-type with long or intermediate length loops, are located in the medulla (Figure 4). In birds, only a small percentage of nephrons (15-25%) contain a loop of Henle (i.e., looped nephrons). Nephrons filter the blood plasma to eliminate waste products, but, in doing so, must not lose needed materials (like glucose) or too much water. Blood enters nephrons via small arteries called afferent arterioles (Figure 5). This blood enters the glomerulus (a collection of capillaries; Figure 6) under high pressure and 'filters' through the walls of the capillaries and the walls of a surrounding structure called a capsule. The filtrate that moves from the glomerular capsule into the proximal tubules is basically plasma without protein (the protein molecules are too large). That filtrate, therefore, contains lots of important substances. In the proximal convoluted tubules, those needed substances such as vitamins and glucose are reabsorbed into the the blood (Figure 7). Even sunbirds, with a diet rich in glucose, are able to reabsorb almost all (98%) of the glucose that filters into the kidney tubules (McWhorter et al. 2004).
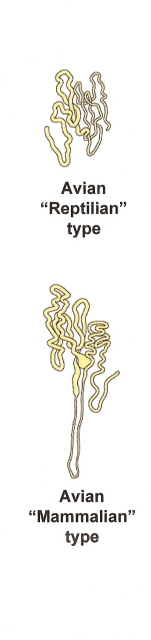 |
Figure 4. Avian nephrons. Most avian nephrons (75-85%) are 'reptilian type', with no loops of Henle. Only 15-25% are 'mammalian type' that have loops of Henle. |
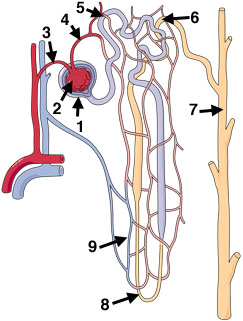 |
Figure 5. Nephron components (mammalian type nephron shown): Plasma is filtered from the glomerular capillaries into the glomerular capsule. Filtrate then travels through the tubules and loop of Henle before entering the collecting duct. |
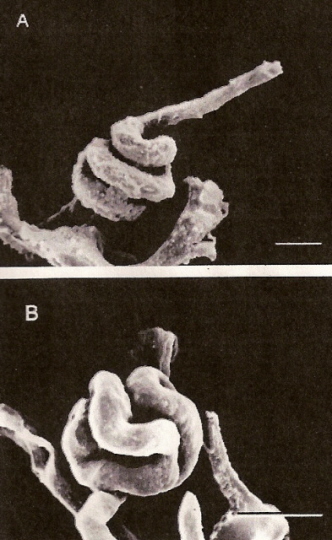
Figure 6. Glomeruli of an Anna's Hummingbird. In some cases (A), the glomerular capillary is twisted
into a spiral (scale bar = 17 micrometers), but more typically (B) is bent into one or two
loops that fold back on themselves (scale bar = 20 micrometers). From: Beuchat et al. (1999).
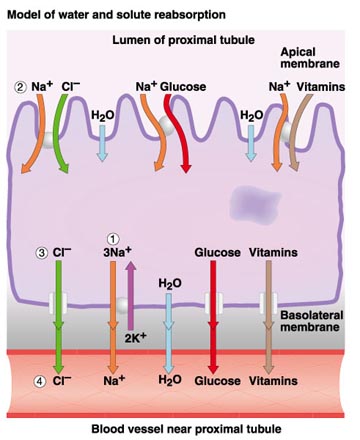
Figure 7. Reabsorption of materials from the proximal convoluted tubule back into the blood.
Image source: http://www.uic.edu/classes/bios/bios100/lecturesf04am/lect21.htm
Other than mammals, birds are the only vertebrates that conserve body water by producing urine osmotically more concentrated than the plasma from which it is derived. However, the ability of birds to concentrate urine is limited compared to mammals. Typically, water-deprived birds produce urine that is 1.4-2.8 times more concentrated than plasma, whereas some mammals can produce urine 20-25 times more concentrated than plasma (but, for most mammals, urine is about 5 - 10 times more concentrated than plasma). This 'concentrating capacity' resides within the medullary cones. Solutes (sodium chloride, or NaCl) are actively transported out the ascending limb of the Loop of Henle (Figure 8), where they become concentrated in the medulla (medullary cones). When urine passes throughout the osmotic gradient in the medulla, water leaves the tubules by osmosis and the urine become concentrated. Because only the looped nephrons contribute to the intramedullary osmotic gradient, the presence of loopless nephrons may limit the ability of the kidneys to produce hyperosmotic urine. Thus, the concentrating ability of avian kidneys is more limited than in mammals.
This reduced capacity of avian kidneys to concentrate urine (compared to mammals) means that more water accompanies the solutes that travel from the kidneys through ureters to the cloaca. Water-deprived birds do have a mechanism for reducing the amount of water leaving the kidneys. In response to dehydration, the pituitary gland releases more of a hormone called arginine vasotocin (AVT) into the blood. In the kidneys, AVT causes a reduction in the glomerular filtration rate (the rate at which plasma filters from the glomeruli into the glomerular capsule; Figure 8) so less water moves from the blood into the kidney tubules. In addition, AVT increases the permeability of the walls of collected ducts to water by opening protein water channels called aquaporins (Figure 8). As the collecting ducts become more permeable, more water moves by osmosis out of the collecting ducts (because of the higher solute concentration in the medullary cones) and can be reabsorbed by kidney capillaries. Studies to date suggest that the extent to which AVT can reduce urine production (and water loss) varies among species, but, in general, AVT is less effective in conserving water than the mammalian equivalent (antidiuretic hormone, or ADH; Nishimura and Fan 2003). Therefore, water-deprived birds tend to produce more urine, and lose more water from the kidneys, than would a similar-sized water-deprived mammal.
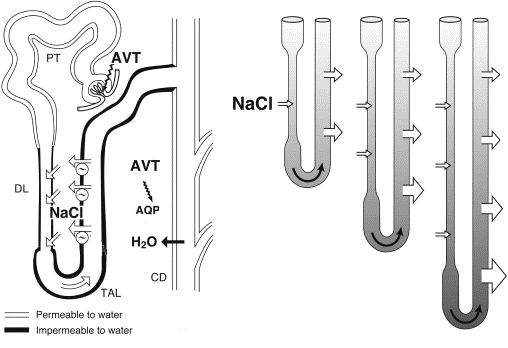
Figure 8. A proposed model for urine concentration in the mammalian-type nephron of birds showing transport of NaCl and water in various nephron segments. Left, NaCl is actively exported out of the thick ascending limb (TAL) and some re-enters the descending limbs (DLs) by simple diffusion (but, despite the resulting high solute concentration, water does not follow because the membrane is not permeable to water). Right: Loops of Henle exhibit some variation in length and more NaCl is transported out of the longer loops of Henle so the osmotic gradient is greatest near the end of the medullary. AVT (arginine vasotocin) is a hormone that helps birds conserve water by reducing the glomerular filtration rate (the rate at which plasma moves from glomeruli in the glomerular capsules) and by increasing the permeability of collecting ducts to water (by opening protein water channels called aquaporins; AQP) (Figure from Nishimura and Fan 2003).
Source: http://faculty.plattsburgh.edu/thomas.wolosz/turbinates.htm
Nasal respiratory turbinates are complex, epithelially lined structures in nearly all birds (and mammals) that act as intermittent countercurrent heat exchangers during respiration. Respiratory turbinates also allow birds to conserve water (by helping to 'dehumidify' air during exhalation).
|
This graph depicts water vapor added to inhaled air (shaded arrow) and water condensate recovered from exhaled air (open arrow). Curves represent water vapor content of fully saturated air (relative humidity [RH] = 100%) and air at 20% saturation. In this example, inhaled air is modified from ambient air temperature and humidity (15°C, 20% RH) to lung air conditions (35°C, 100% RH). After cooling by the turbinates, exhaled air is still saturated but at only 18°C. As a result, about 66% of the water vapor originally added to inhaled air is recovered (From: Hillenius and Ruben 2004). |
THE SWEET CHALLENGE by

In the kidney, water is filtered out of blood by specialized structures called glomeruli, and some of the eliminated water is later reabsorbed in the nephron and collecting duct. The researchers set out to test how these processes respond to water intake in Palestine Sunbirds. Although following the birds around and measuring their nectar intake is difficult, McWhorter and his colleagues came up with an ingenious solution to the problem. They discovered that the birds adjust the amount they consume according to the concentration of sucrose solutions they are fed: the more dilute the solution, the higher the volumes ingested. In this way, the team could vary the bird's water intake and measure the rates of renal filtration and reabsorption.
McWhorter explained that when the team began investigating this nectarivorous bird's approach to fluid management, it was thought that renal filtration changes according to water status; decreasing in response to water shortage, but increasing only moderately as the birds take on water. But this was based on ideas developed for birds that do not regularly cope with a large intake of water. McWhorter and his team also knew that when the birds are on dilute diets, water is shunted through the gut without being absorbed. So, how would Palestine Sunbirds' kidneys cope?
The team found that renal filtration is not exceptionally sensitive to water loading in sunbirds; it increased only slightly in response to a dramatic decrease in sucrose concentration. On the other hand, the fractional water reabsorption - a measure of the proportion of the eliminated water that is reabsorbed by the kidney - dropped significantly when the birds were on the most dilute diet. The sunbirds' kidney responds to the elevated water levels by decreasing reabsorption, rather than by raising the filtration rate.
The team also found that the glucose and osmotic concentrations in the final excreted fluids were significantly lower than those in the ureteral fluids released by the kidney. Because the gut and urinary tracts of birds join at the cloaca, the researchers conjecture that the dietary water that shunts through the gut might have diluted the ureteral fluids. They conclude that Palestine Sunbirds deal with large amounts of water intake by not absorbing it in the first place.
From an economical standpoint this makes sense, as eliminating water by increasing renal filtration rate can be energetically costly for birds. Sugar and other metabolites lost during filtration may only be retained by reabsorption, possibly overwhelming the kidney's ability to prevent solute loss. But how the gut could absorb nutrients without taking in dietary water is still a mystery, as the two processes normally come hand in hand (Click on the photo to check out Peter Jones' website).
Hummingbird tongues
An important part of the diet of all birds is protein. Proteins are composed of subunits called amino acids, and those amino acids are sometimes used as a source of energy or are converted into fats or carbohydrates. When amino acids are used for energy or converted to fats or carbohydrates, the amine (NH2) group must be removed. These amine groups are toxic and must be eliminated. Some organisms excrete these nitrogenous wastes as ammonia (e.g., aquatic animals like bony fishes and amphibians) or urea (e.g., terrestrial amphibians and mammals). Birds (and reptiles) excrete these wastes primarily as uric acid. Although excreting nitrogenous wastes as uric acid has its advantages (e.g., not very toxic, not soluble in water so it can be excreted without using lots of water, and it can be stored in eggs without damaging embryos), uric acid is a more complex molecule than either ammonia or urea (Figure 9) and synthesizing it requires more energy
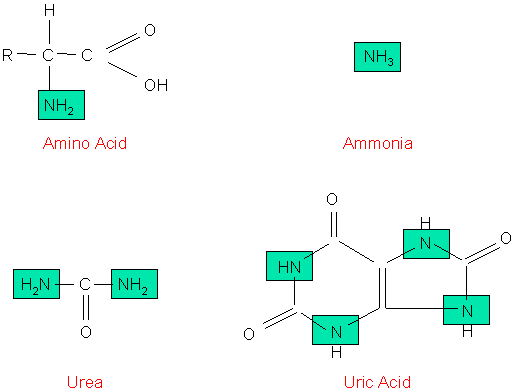
Molecular structure of a typical amino acid, ammonia, urea, and uric acid.
Source: http://faculty.clintoncc.suny.edu/
The homeostasis of fluid and ions in birds involves several organ systems (Figure 10) and is a more complex phenomenon than in other vertebrates. In birds, the kidneys and lower gastrointestinal tract (cloaca, rectum, and ceca) are involved in the regulation of extracellular fluid composition. Many birds also have functional salt glands (see below).
Figure 10. Osmoregulatory organs of birds (Hughes 2003).
As noted above, the avian kidney has a limited capacity for the conservation of body water and electrolytes via elimination of hyperosmotic urine. This low capacity to concentrate urine is not a liability because urine formed by the kidneys travels along the ureters into the cloaca (Figure 11). From there, it may move by retrograde peristalsis into the lower intestine (colon) and cecae. Fluid from the upper gastrointestinal tract also enters the cloaca. Therefore, the cloaca receives an influx of water from the kidneys and the small intestine. The influx of water into the cloaca can be reabsorbed through the epithelium of the lower intestinal tract to maintain hydration. In the lower intestine and cecae, water and sodium chloride are reclaimed by the process of sodium-linked water reabsorption (Figure 11). In other words, positively-charged sodium ions are actively transported out of the intestine and negatively-charged chloride ions follow. Water then follows by osmosis. Uric acid is, as a result, concentrated and excreted as a relatively dry mixture with feces (Hildebrandt 2001).
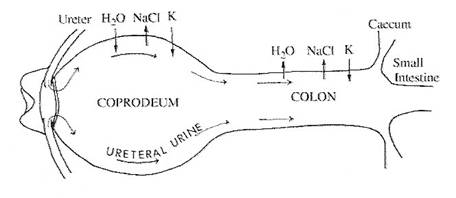
Figure 11. Diagram of the cloaca and lower intestine of a domestic chicken (Gallus gallus).
Arrows indicate the net movements of water and electrolytes (NaCl and K +) as well as the
retrograde movement of ureteral urine (urine transported from the kidneys to the cloaca through the ureters). Initially, the solutes in the urine cause water to move by osmosis out of the surrounding tissues and into the coprodeum (the section of the cloaca adjacent to the colon or large intestine). After being transported by peristalsis into the colon, however, NaCl is transported out of the colon and water follows the concentration gradient (osmosis) and is reabsorbed (Laverty and Skadhauge 2008).
The predominate form in which nitrogen is excreted by birds (uric acid) requires little water for excretion because it isn't very soluble in water. However, it does require a significant amount of protein to maintain it in a colloidal suspension in the urine (i.e., urine spheres; Figrue 12). The source of some of this protein is the plasma, as significant amounts pass through the glomerular filtration barrier. This protein is not lost because it is broken down when the urine enters the lower colon (Goldstein et al. 1999). In the colon, the composition of the urine is altered in several ways. The urine spheres are broken down, as is the protein that aided the formation of those spheres. Much of this degradation is accomplished by bacteria. The amino acids or peptides that result are either used by the bacteria or absorbed by the epithelium of the colon (Braun 1999).
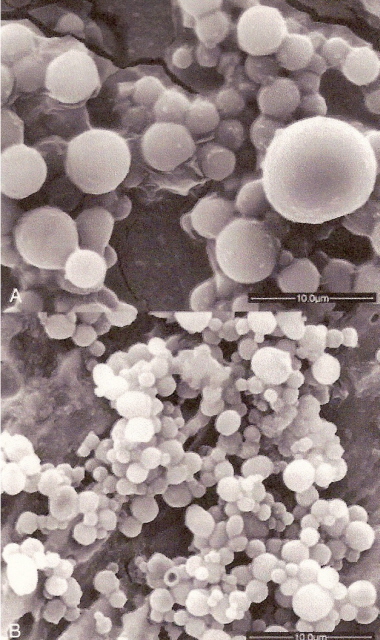
Figure 12. "Urine spheres" showing differences in size.
Top photo = domestic chicken;
Bottom photo = Wood Thrush.
(From: Casotti and Braun 2004).
Possible role of ceca in fluid & electrolyte homeostasis -- Because birds do not have a urinary bladder, the kidneys and lower gastrointestinal tract must function in concert to maintain fluid and electrolyte homeostasis. In birds, urine is conveyed to the cloaca, and moved by reverse peristalsis into the colon and digestive ceca. Digestive ceca have been well studied for non-passerine birds and have been shown to absorb substrates and water. The ceca of passerine birds have been suggested to be non-functional because of their small size. Three-dimensional reconstruction of the ceca of House Sparrows (Passer domesticus) from serially-sectioned tissue showed that the ceca have a central channel with a large number of side channels. Electron micrographs indicated that all of the channels are lined by epithelial cells with a very dense microvillus brush border as well as a region densely packed with mitochondria just below the brush border. It is possible that the row of mitochondria below the brush border is present to provide ATP to power substrate transport. Although the importance of small ceca for fluid homeostasis remains to be determined, these data suggest that the small ceca of passerine birds may function in fluid and electrolyte (e.g., sodium) homeostasis (Reyes and Braun 2005).
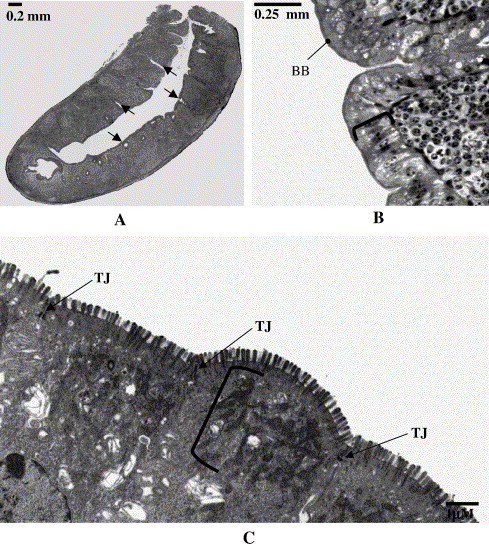
Light micrograph and electron micrographs of a House Sparrow cecum. A) A light micrograph of a cecum cut in sagittal section.
The image shows one central channel with a proximal opening to the colon (upper right of section). Side channels (arrows) can be seen branching
from the central channel. B) Higher power light micrograph of a cecum. The image illustrates the columnar cells that line the channels (bracket) with their
well developed brush border (arrow). C) Electron micrograph of a House Sparrow cecum. The image shows a portion of the epithelial cells that line channels
of the ceca. Evident is a dense microvillus brush border on the apical surface of the cells and tight junctions (TJ) between the cells. Just below the brush border is
a very dense layer of mitochondria (bracket). 7400 × (From: Reyes and Braun 2005).
As described above, the colon and ceca of birds can substantially modify the urine that initially enters the cloaca (coprodeum). The lower section of the bird gastrointestinal tract, by the same mechanisms, alters ‘feces’ coming from the small intestine. As a result, the composition of bird droppings can differ dramatically from the composition of the urine and feces that initially entered the colon or large intestine (Figure 13). Depending on diet and access to water, droppings may have considerably less water, solutes (sodium and potassium), and uric acid (or urate) than the original urine and feces (Figure 13). Of course, if a bird consumes more of these substances than needed, the composition of droppings will differ and more water, solutes, and urates can, as needed, be excreted in the droppings.
The coordinated action of the kidneys, lower GI tract, and the salt glands (see below) in the regulation of fluid and ion balance is a classic example of the integration of organs required to maintain homeostatic balance. No single organ appears to have an outstanding capacity to conserve ions and water, but instead they all function in concert to maintain total body fluid homeostasis to allow birds to inhabit a wide range of environments (Braun 1996).
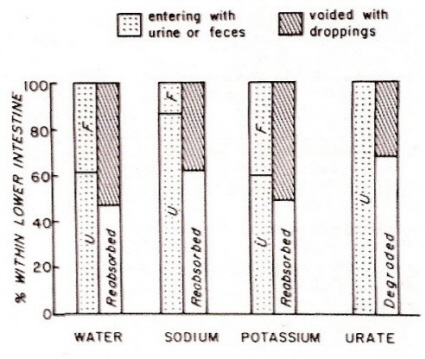
Figure 13. Modification of urine and feces in the lower GI tract (colon and ceca) of birds.
(Source: http://eebweb.arizona.edu/courses/Ecol437/EldonBraun_Ecol%20lect%2004.pdf)
Can birds be ammonotelic?? -- Most birds are thought to excrete nitrogen as uric acid and, therefore, are referred to as uricotelic. Uric acid is a relatively non-toxic nitrogen end product. It is relatively insoluble and hence excreted with little water. Uricotely, however, is costly. More energy is needed to excrete a unit of waste nitrogen as uric acid than as urea or ammonia. In contrast to uric acid, ammonia is highly soluble, cheap to synthesize, but fairly toxic. It can only be used as a nitrogenous waste by animals with high rates of water turnover that permit almost  continuous elimination, such as in aquatic animals (Tsahar et al. 2005).
continuous elimination, such as in aquatic animals (Tsahar et al. 2005).
Preest and Beuchat (1997) suggested that it might be advantageous for birds that ingest large amounts of dilute, protein-poor nectar to shift from uricotely to ammonotely. Thus, ammonia can be voided rapidly, and the costs of synthesizing urates can be reduced. Preest and Beuchat (1997) called the shift from uricotely to ammonotely in hummingbirds `facultative ammonotely' In their study on Anna's Hummingbird (Calypte anna; see photo to the right), Preest and Beuchat concluded that these birds were facultatively ammonotelic. At low ambient temperatures and high water intakes, Anna's Hummingbirds excreted more than 50% of their total nitrogen excretion as ammonia (i.e. they became ammonotelic), whereas at higher ambient temperatures and lower food intake they were uricotelic). Thus, Preest and Beuchat (1997) proposed that high energy demands and high water fluxes favor ammonotely.
Subsequently, Roxburgh and Pinshow (2002) found that the Palestine Sunbirds also switched from uric acid to ammonia excretion under some conditions.
However, Roxburgh and Pinshow
(2002) noted that in sunbirds with high water intake, the concentration of urate was higher in the ureters (the tubes that carry urine from the kidneys to the cloaca) than in excreta. They argued that ammonotely in Palestine Sunbirds was only 'apparent' because it was not a result of excessive excretion of ammonia, but rather the result of a reduction in excreted urate resulting from post-renal modification of urine. Recently, Tsahar et al. (2005) then found that Yellow-vented Bulbuls (Pycnonotus goiavier), a frugivorous species, appeared to switch from uricotely to ammonotely when they ingested large amounts of water and when their protein intake was low.
These authors, like Roxburgh and Pinshow (2002) found that protein concentration was lower in excreta than in ureteral urine, and hypothesized that some of the protein associated with urate spheres was digested in the lower intestine and recovered.
Why would it be advantageous for birds to recover a nitrogenous metabolic waste? Although uric acid is considered primarily a nitrogenous waste, it also has a major function as a powerful antioxidant in both birds and mammals (Tsahar et al. 2005).
So, can birds be ammonotelic? Apparently, yes. In some cases, as with Anna's Hummingbirds, ammonotely may be a response to the ingestion of lots of water (facultative ammonotely). In other cases, ammonotely may simply be 'apparent', with urine produced by the kidneys being urotelic but the actual excreta being ammonotelic because of reabsorption of uric acid in the lower intestine or colon prior to defecation.
Because the kidneys of birds cannot produce a hypertonic urine (with lots of ions like sodium), the excretion of excess salt is a potential problem. Even quicker than humans, birds would be severely dehydrated after drinking saltwater and ingesting salty food. However, many species of birds, especially marine birds and shorebirds, can drink seawater as their only source of water. This is possible because these birds have another way (other than the kidneys) to eliminate excess salt - salt glands.
Salt glands of birds likely evolved from nasal glands of reptiles, probably in the late Paleozoic. They lie immediately under the skin in supraorbital depressions of the frontal bone in the skull of Charadriiform birds, but in other groups they may be located above the palate or within the orbit of the eye. Skulls of fossil birds, Ichthyornis and Hesperornis, have similar depressions (Figure 14), suggesting these birds lived in marine habitats. The salt glands of marine birds (and some falconiform and desert birds) secrete excess NaCl via the salt glands using less water than is consumed, which generates free water (Hughes 2003).

Figure 14. Hesperornis skull (note depression above eye socket where salt gland would be located)
Salt glands have been reported in several avian orders (Spheniciformes, Procellariformes, Charadriiformes, Pelecaniformes, Anseriformes, and Phoenicopteriformes). Even though most studies of osmoregulation in birds have been conducted with marine taxa, nasal secretions are not to be restricted to these species. The presence of functional salt glands has been documented in several terrestrial orders. For example, Roadrunners (Geococcyx californianus) and Savanah Hawks (Heterospizias meridionalis), have active salt glands and can produce hypertonic secretions in response to their protein-rich diets. Although these species are not stressed by high saline load, the active secretions of salt gland allows them to minimize water losses. Other desert birds, such as the Sand Partridge (Ammoperdix heyi) and the Ostrich (Struthio camelus), have functional salt glands that are stimulated in response to high temperature. Thus, salt glands are not restricted to birds that live in saline or maritime habitats, but are also present in some terrestrial forms that consume little water (Sabat 2000).
Salt glands have a system of countercurrent blood flow to remove and concentrate salt ions from the blood (Figures 15 and 16). The paired, crescent-shaped glands each contain several longitudinal lobes approximately 1 mm in diameter and each lobe contains a central duct from which radiate thousands of tubules enmeshed in blood capillaries. These tiny capillaries carry blood along the tubules of the gland, which have walls just one cell thick and form a simple barrier between the salty fluid within the tubules and the bloodstream. It is here that salt excretion occurs.
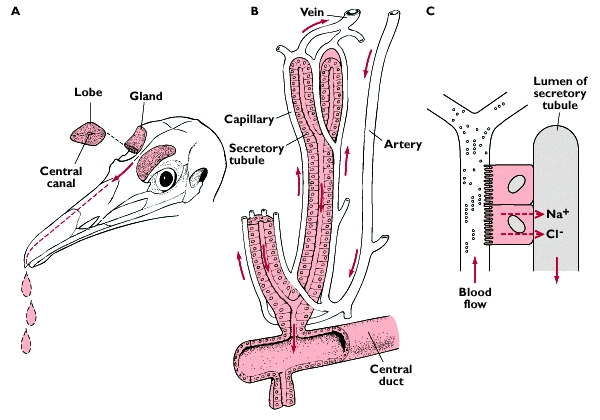
Source: http://www.kcl.ac.uk/ip/christerhogstrand/courses/hb0223/water&io.htm
Figure 15. When a bird drinks seawater, sodium enters the blood plasma from the intestine and the solute concentration of the blood plasma increases.
This causes water to move out of cells (osmosis), increasing the extracellular fluid volume (ECFV). The increases in blood plasma solute concentration
and ECFV stimulate salt gland secretion (Hughes 2003).
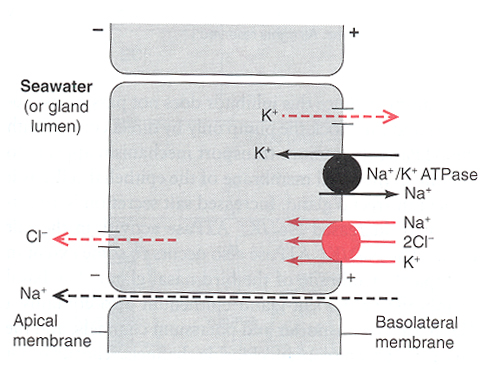
Figure 16. It has been proposed that the enzyme Na+-K+-ATPase provides energy (by breaking down ATP) for the co-transport of
Na+ and Cl- through the folded basal membranes. Chloride then moves passively through the apical membrane and Na+ flows
between the principal cells, through the tight junctions and into the tubular lumen. Water movement may follow solutes via the cellular
or paracellular route to yield a hypertonic fluid composed primarily of NaCl together with smaller amounts of K+ and traces of other ions (Butler 2002).
Black sphere = active transport; red sphere = cotransport of sodium, potassium, and chloride ions.
Convergent evolution of kidney sizes and supraorbital salt glands for birds living in saline habitats-Only a small number of avian species inhabit salty environments. To understand how they adapted, Chiu et al. (2024) examined the evolution of kidney sizes, supraorbital salt glands (SSGs), and the utilization of salty habitats across 230 species spanning 25 avian orders. Phylogenetic analysis indicates that SSGs, large kidneys, and thriving in salty habitats emerged convergently in birds. Analysis also revealed that species possessing SSGs and large kidneys tended to move from low-to high-salinity environments, while others moved in the opposite direction. However, habitat salinity also influenced kidney evolution; lineages found in high-salinity environments tended to develop larger kidneys than those in low-salinity environments. These results suggest that SSGs and large kidneys may have evolved through adaptation to high salinity environments. Overall, habitat conditions and physiological traits influenced avian adaptation to salty environments in a reciprocal manner. These results shed the new light on the evolutionary mechanisms underlying functional diversity in birds.
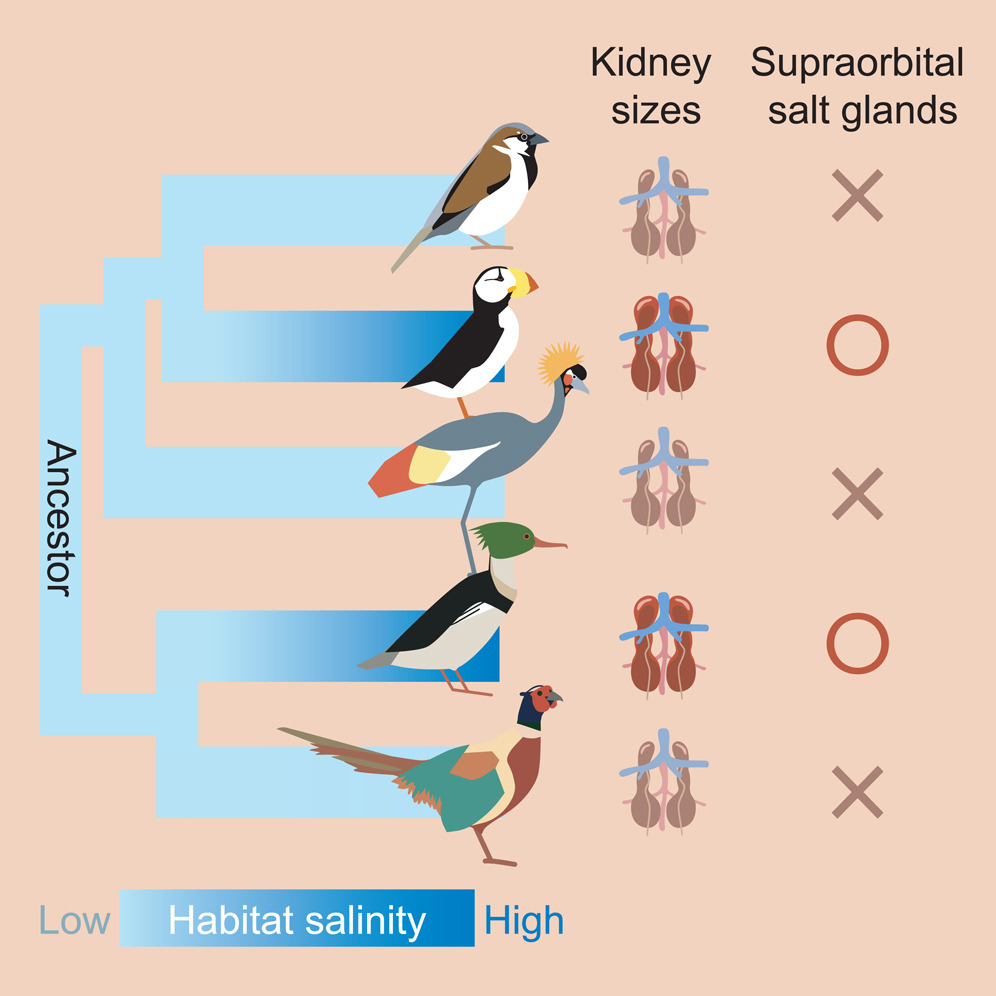
Species of birds that occupy salty environments, like puffins and mergansers
have salt glands, and also have larger kidneys than species found in low salinity
environments. Darker kidneys are larger and the symbol O indicates
the presence of salt glands (X = no salt gland) (From: Chiu et al. 2024).
How Seabirds Drink Saltwater and Survive!
Salt gland dissection
Do birds sneeze? How some seabirds cope with their high-salt diets
Beuchat, C. A., M. R. Preest, and E. J. Braun. 1999. Glomerular and medullary architecture in the kidney of Anna's Hummingbird. Journal of Morphology 240:95-100. Braun, E. J. 1999. Integration of organ systems in avian osmoregulation. Journal of Experimental Zoology 283:702-707. Butler, D. G. 2002. Hypertonic fluids are secreted by medial and lateral segments in duck (Anas platyrhynchos) nasal salt glands. Journal of Physiology 540.3: 1039-1046. Casotti, G. and E. J. Braun. 2004. Protein location and elemental composition of urine spheres in different avian species. Journal of Experimental Zoology 301A: 579-587. Chiu et al. 2024. Convergent evolution of kidney sizes and supraorbital salt glands for birds living in saline habitats. iScience 27: 109169. Conradie, S. R. and and M. T. Freeman. 2026. Classifying avian drinking behaviour: ecological insights and implications
in a changing world. Biological Reviews, https://doi.org/10.1002/brv.70150. Duke, G. E., A. A. Degen, and J. K. Reynhout. 1995. Movement of urine in the lower colon and cloaca of Ostriches. Condor 97:165-173. Goldstein, D.L., V. Reddy, and K. Plaga. 1999. Second messenger production in avian medullary nephron segments in response to peptide hormones. Am J Physiol Regul Integr Comp Physiol 276: R847–R854. Heidweiller, J. and G. A. Zweers. 1990. Drinking mechanisms in the Zebra Finch and the Bengalese Finch. Condor 92:1-28. Hildebrandt, J.-P. 2001. Coping with excess salt: adaptive functions of extrarenal osmoregulatory organs in vertebrates. Zoology 104:209-220. Hillenius, W. J., and J. A. Ruben. 2004. The evolution of endothermy in terrestrial vertebrates: who? when? why? Physiological and Biochemical Zoology 77: 1019-1042. Hughes, M. R. 2003. Regulation of salt gland, gut and kidney interactions. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 136: 507-524. Klaassen, M. 1996. Metabolic constraints on long-distance
migration in birds. Journal of Experimental Biology 199: 57-64. Laverty, G., and E. Skadhauge. 2008. Adaptive strategies for post-renal handling of urine in birds. Comparative Biochemistry and Physiology, Part A 149: 246-254. McSweeney, T., and M. K. Stoskopf. 1989. Selected features of the abdominal and thoracic anatomy of the Brown Pelican (Pelecanus occidentalis). Journal of Zoo and Wildlife Medicine 20: 184-190. McWhorter, T. J., C. Martínez del Rio, B. Pinshow, and L. Roxburgh. 2004. Renal function in Palestine Sunbirds: elimination of excess water does not constrain energy intake. J. Exp. Biol. 207: 3391-3398. Nishimura, H. and Z. Fan. 2003. Regulation of water movement across vertebrate renal tubules. Comp. Biochem. Physiol. - Part A 136: 479-498. Reyes, L. and E. J. Braun. 2005. The functional morphology of the english sparrow cecum. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 141: 292-297. Roxburgh, L. and B. Pinshow. 2002. Ammonotely in a passerine nectarivore: the influence of renal and post-renal modification on nitrogenous waste product excretion. J. Exp. Biol. 205:1735 -1745.
Ruaux, G., et al. 2023. Drink safely: Commmon Swifts dissipate mechanical energy to decrease flight speed before touch-and-go drinking. Journal of Experimental Biology, https://doi.org/10.1242/jeb.244961 Sabat, P. 2000. Birds in marine and saline environments: living in dry habitats. Rev. Chil. Hist. Nat. 73: 401-410. Schmidt-Nielsen, K. 1997. Animal physiology: adaptation and environment,
5th ed. Cambridge University Press, Cambridge, UK. , L., J. Klandorf, and P. Yancey. 2005. Animal physiology - from genes to organisms. Brooks/Cole, Pacific Grove, CA. Williams, J. B., W. R. Siegfried, S. J. Milton, N. J. Adams, W. R. J. Dean, M. A. du Plessis, S. Jackson, and K. A. Nagy. 1993. Field metabolism, water requirements, and foraging behavior of wild Ostriches in the Namib. Ecology 74: 390-404.
Braun, J. 1996. An overview of osmoregulation in birds. Abstract - VI International Symposium of avian endocrinology.
Chateau Lake Louise, Alberta, Canada.
Preest, M. R. and C. A. Beuchat. 1997. Ammonia excretion by hummingbirds. Nature 386:561 -562.