|
Ornithology Avian Reproduction:
|
 |
|
Ornithology Avian Reproduction:
|
 |
Clutch size
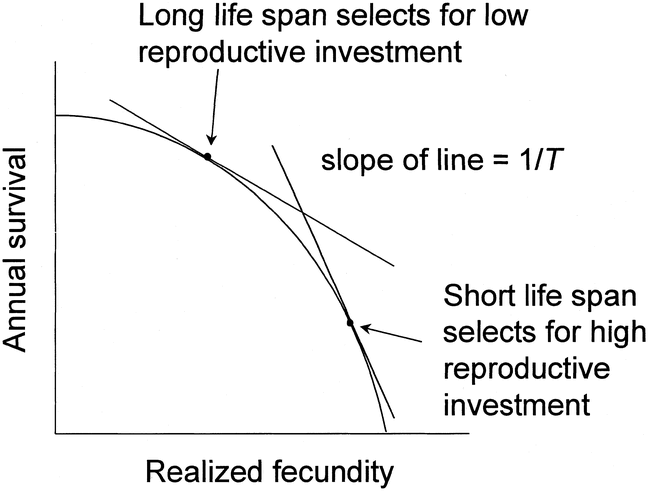
The optimization of the trade-off between annual adult survival and annual fecundity. The bounded area within the curve represents the set of
possible phenotypes, among which those potentially having maximum fitness are represented on the periphery. The adaptive function is a line running through
combinations of fecundity and survival having equal fitness and just tangent to the fitness set. The tangent point is the phenotype that maximizes evolutionary fitness.
The slope of the line (−1/T) is the negative value of the inverse of the average age (T) of a female at the birth of her offspring. Thus, the slope is related to the negative of
the adult mortality rate (From: Ricklefs 2000).
The considerable interspecific variation in typical clutch sizes indicates that natural selection has favored different reproductive strategies for species that vary in lifespan, habitat use and quality, geographic location, and other factors. Determining the extent to which different factors have acted via natural selection to influence the evolution of clutch sizes in different species is clearly difficult because of the many factors involved. However, one of those factors is clearly life span.
Time, energy, and other resources are limited and must be allocated among competing demands (Ricklefs 2000). The chances of surviving the various risks associated with reproduction will likely decrease as parental investment increases because time and resources that could be allocated to adult maintenance and avoidance of predation are instead devoted to offspring. Because natural selection favors individuals that optimize lifetime reproductive success (and not reproductive success during a single breeding attempt or breeding season), the optimal investment in a particular breeding attempt is influenced by life span. In other words, individuals in species with a shorter life expectancy may maximize their life-time fitness by investing more in reproduction (e.g., larger clutches); individuals in species with longer life spans enhance their fitness by investing less in reproduction.
Fecundity (or life-time fitness) is, therefore, related to and greatly influenced by annual survival. To illustrate, in the Figure above, the bounded area represents the potential fitness of all possible phenotypes with respect to realized fecundity and reproductive survival of parents. Because, in this graph, fitness increases with distance from the origin of the graph (a combination of higher fecundity and higher survival), all points inside the bounded area represent less fit phenotypes and the outer perimeter of that area represents optimum fitness. As a result, natural selection should lead to an optimum phenotype somewhere on the perimeter. The optimum point is tangent to a line (the “adaptive function”) whose slope is determined by the annual adult survival rate. Thus, when the likelihood of adults surviving due to factors other than reproductive risk is higher, the adaptive function has a shallower slope and is tangent to the adaptive function at a lower fecundity, but higher adult survival rate (Ricklefs 2000).
Remember first, if this is unclear, that slope represents the relationship between how much a line rises relative to how long it is or, for a line with a negative slope, how much a line falls relative to its length. Compare an albatross with a possible life span of up to 40 years that starts breeding when 10 to a chickadee with a possible life span of about seven years that starts breeding when one year old. For the albatross, the average number of breeding seasons (assuming a full life span) is 25 and, for the chickadee, four. For the albatross, the slope that maximizes fitness is – 1/25 and, for the chickadee, -1/4. In other words, the slope of the line for the albatross is much shallower and, therefore, its long life span selects for lower investment in each breeding attempt. The much shorter life span of a chickadee means that selection favors more investment in each breeding attempt.
Parental investment and sex allocation in a wild bird (House Wren) by Emerson Keith Bowers
| Age-specific reproductive success has been demonstrated in many species. Three hypotheses have been raised to explain this general phenomenon: the experience hypothesis based on age-specific reproductive experience, the effort hypothesis based on age-specific reproductive effort, and the selection hypothesis based on progressive disappearance of phenotypes due to variation in individual productivity and survival. Mauck et al. (2004) used data from a long-term study of Leach's storm-petrels (Oceanodroma leucorhoa) to present a single test of mutually exclusive predictions about the relationship between early breeding success and longevity. There should be no correlation between early breeding success and longevity under the experience hypothesis, a negative correlation under the effort hypothesis, and a positive correlation under the selection hypothesis. The authors found a significant (P < 0.0001) positive relationship between success in the first two breeding attempts and longevity in this population of long-lived seabirds, strongly suggesting that low-productivity parents were also less likely to survive early breeding. These data provide some of the strongest support to date for the selection hypothesis. |
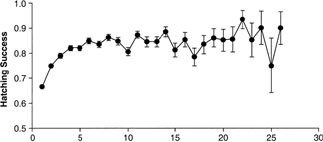
Age (yrs) The relation between hatching success and breeding age in Leach's Storm-Petrels on Kent Island, New Brunswick, Canada, 1962–1995 (N = 18,347).
|
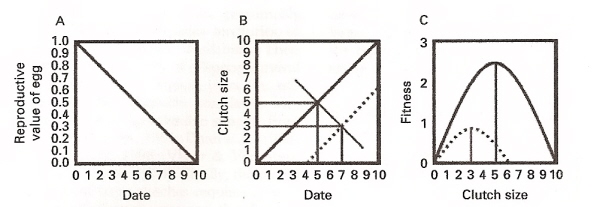
Hypothesis for the seasonal decline in clutch size at higher latitudes based on the seasonal decline in the reproductive value of the eggs
and on variation in female quality or condition. (A) The reproductive value of an egg declines seasonally because the probability of offspring
recruitment (surviving until reproducing) declines
with hatch date. (B) Attaining the physical condition needed to lay a clutch of a given size requires
time, and more time available means a larger clutch is possible. However, at a given date, females of higher quality or in better condition (thick solid line)
lay more eggs than those of lower quality or worse condition (broken line). (C) The product of the reproductive value of a single egg and the number of
eggs yields a fitness curve maximized by some intermediate clutch size. The clutch size that maximizes fitness is smaller for a late-breeding attempt (broken line)
than for an early breeding attempt (solid line), giving rise to a seasonal decline in optimal clutch size (thin, black, diagonal line in B)
(From: Rowe et al. 1994, Sockman et al. 2006).
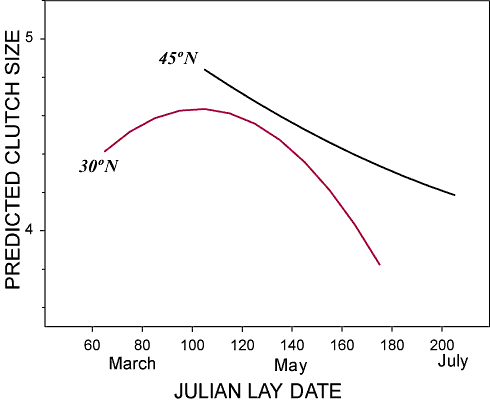
Eastern Bluebird clutch size as predicted from a model
based on 945 clutches. The model
represents values for the year 1997 at 80° W and
at 30° N and 45° N. Clutch sizes farther
south are small early
in the season, increase to a midseason peak, and become smaller
again late in the season.
Clutches farther north decline throughout the season from an initial high.
The Julian lay date expresses date as days since January
1 (From: Dhondt et al. 2000).
Latitudinal variation in clutch sizes
Despite the large range in clutch sizes among different species of birds, more than half of all birds lay clutches of 2 or 3 eggs (mode = 2, median = 2.8; Jetz et al. 2008). Examination of the frequency distribution of avian clutch sizes reveals a skewing to the right (Figure below), and this is due primarily to the relatively large clutches of north temperate species (Figure below; Jetz et al. 2008). Thus, an important question is why clutch sizes exhibit such latitudinal variation and, specifically, why clutch sizes tend to be larger in temperate areas (or, depending on your frame of reference, why clutch sizes are smaller in tropical areas).
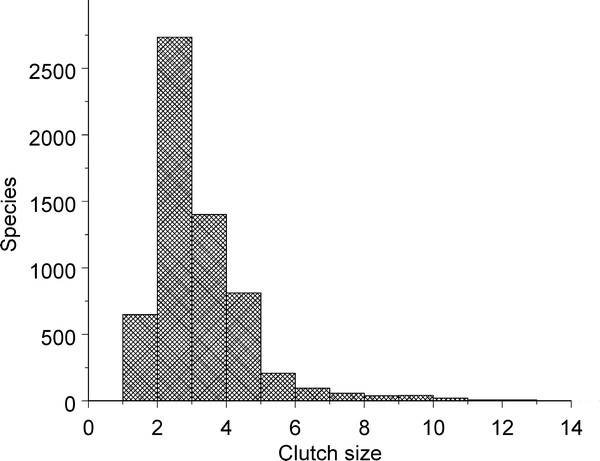
Global variation in the mean clutch sizes of 5,290 species of landbirds (six species with mean clutch sizes greater than 14 eggs not included) (Jetz et al. 2008).
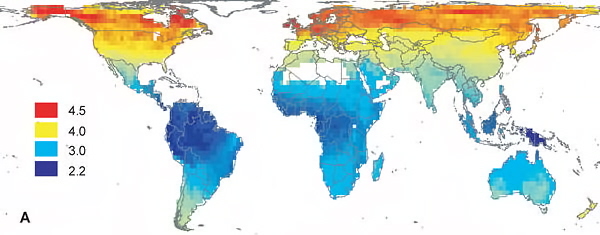
Geographic patterns in mean clutch size for 5,290 bird species (Jetz et al. 2008).
Birds in tropical areas commonly lay about half as many eggs as those in north temperate areas. Lack (1947) proposed that latitudinal variation in clutch sizes was the result of differences in food availability. This food limitation hypothesis posits that clutch size is determined by food supply, with natural selection favoring clutch sizes that correspond to the number of viable young that the parent(s) can successfully raise, and the scarcity of food in tropical habitats limits clutch sizes. Another hypothesis, the nest predation hypothesis, proposes that small clutch sizes are favored in the tropics because there are more predators in the tropics and predators are more likely to locate nests with larger clutches and more young because more young mean increased rates of food delivery by adults and a greater likelihood that, with the increased activity, predators will locate nests. High rates of nest predation may also select for smaller clutches to reduce the investment in any single nesting attempt (Slagsvold 1982, Martin 1995). Field studies have provided little support for either of these hypotheses (Martin et al. 2000, Stutchbury and Morton 2008).
Clutch sizes of birds occupying aseasonal environments where there is little seasonal variation in temperature are smaller than those of birds inhabiting environments where temperatures are more variable. Highly seasonal environments can cause increased adult mortality (Ricklefs 1997) because birds must survive periods of lower temperatures and reduced food availability or because birds must take risks associated with migration (Jetz et al. 2008). However, seasonal environments also have periods when food availability is very high. This combination of abundant food during the breeding season and increased mortality during the non-breeding period has favored the evolution of larger clutches in seasonal environments; larger clutches can be produced and more young raised to fledging because of the increased availability of resources and producing more young enhances parental fitness because it increases the likelihood that some will survive the period of increased mortality during migration or, for resident birds, during periods of reduced food availability. The greater the degree of seasonality, or the greater the fluctuation in resource availability, the greater is the tendency for larger clutches. Seasonality increases with increasing latitude as do avian clutch sizes. However, seasonality also varies in some cases with longitude. For example, western Europe is less seasonal than eastern Europe and, as expected given the effect of seasonality on clutch size evolution, birds in eastern Europe tend to have larger clutches than birds in western Europe (Bell 1996). In addition, seasonal variation in resource availability for migrants is lower than for residents. Assuming that migration costs (and associated mortality) are not too high, migratory birds might be expected to have smaller clutches than residents that overwinter. This appears to be the case (Böhning-Gaese et al. 2000).
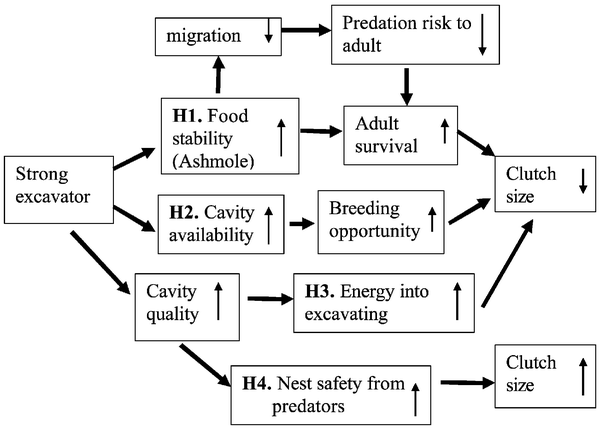
Potential pathways explaining the evolution of clutch size in excavating birds, showing causal links from left to right.
The arrows within the boxes indicate whether that factor increases or decreases in response to the causal agent preceding it in the
pathway, e.g., increasing food stability causes increasing adult survival. The figure encapsulates the following hypotheses:
(H1) stability of food resources, (H2) nest site limitation, (H3) energy costs of excavation, and (H4) predation risk on the nest. Other factors
that affect adult survival independent of excavation ability may also influence clutch size.
Clutch sizes of cavity-excavating birds -- There are two major competing hypotheses for variation in clutch size among cavity-nesting species. The nest site limitation hypothesis postulates that nesting opportunities are more limited for weak excavators, which consequently invest more in each breeding attempt by laying larger clutches. Alternatively, clutch size may be determined by diet; the clutch sizes of strong excavators may be smaller because they are able to specialize on a more seasonally stable prey. Wiebe et al. (2006) built a conceptual model that integrated hypotheses for interspecific variation in clutch size and tested it with comparative data on life-history traits of woodpeckers (Picidae) and nuthatches (Sittidae). In most analyses, diet explained more variation in clutch size among species than did propensity to excavate. Migratory status was positively associated with clutch size, but was difficult to distinguish from diet because resident species consumed more bark beetles (a prey available in winter) and had smaller clutches than migratory species. The literature suggests that cavities are not limited in natural, old-growth forests. Although Wiebe et al.'s (2006) data do not rule out nest site limitation, the authors concluded that annual stability of food resources has a larger impact on the evolution of clutch sizes in excavators than does limitation of nest sites.
Syrian Woodpecker (Dendrocopos syriacus)
Evolution of clutch sizes
Possible factors involved:

Source: http://www.bbc.co.uk/nature/birds/birdpages/35.html |
The cost of egg production: increased egg production reduces future fitness in gulls -- Measurements of costs of reproduction are essential for our understanding of the evolution of reproductive effort. While the effects of increased chick-rearing effort on subsequent survival and fecundity have been relatively well studied experimentally, costs associated with increased egg-production effort have received less attention. Nager et al. (2001) experimentally increased the egg-production effort of individually-marked Lesser Black-backed Gulls (Larus fuscus) and followed their breeding performance in the next year. In the season following increased egg production, females, but not males, were less likely to be resighted in the study plot and those that did return were less likely to produce a clutch compared to control birds. In addition, those experimental females that did breed invested less in egg production the following season. Thus, this study provides evidence that there is an inter-brood trade-off between current egg-production effort and future fitness in birds. |

Predation risk and clutch size -- Clutch size reduction under high risk of nest predation has been hypothesized to be adaptive for at least two reasons. First, when nest predation increases with clutch size, smaller broods will shorten the period when the nest is susceptible to nest predators and reduce the number of nest visits that could attract the attention of predators. Second, if parental survival declines with clutch size, then a reduction in clutch size will improve parental survival prospects and future reproduction, thereby spreading the risk of nest predation between broods and ultimately increasing lifetime reproductive success. Despite these predictions being consistent with life-history theory, there is little experimental support for nest predation affecting clutch size variation in birds. This is surprising because many theoretical and experimental studies on other taxa provide evidence for predator-induced life-history shifts through phenotypic plasticity. Past work on bird clutch sizes has mainly involved intra- and interspecific comparative analyses, which provides important insights into factors associated with clutch size variation, but does not necessarily infer a causal relationship. Eggers et al. (2006) manipulated perceived risk of nest predation in Siberian Jays (Perisoreus infaustus) using playback of a mixture of calls by corvid nest predators in the vicinity of nest sites. In response to being exposed to this acoustic cue simulating increased risk of nest predation, the jays chose a nest site offering more protective covering and reduced clutch size. This is the first experimental demonstration of clutch size adjustment and nest site selection as a result of phenotypic plasticity in an open nesting passerine reflecting a facultative response to the perceived risk of nest predation (Photo source: http://www.animalpicturesarchive.com).
Northern Harrier taking eggs from a Streaked Horned Lark (Eremophila alpestris strigata) nest
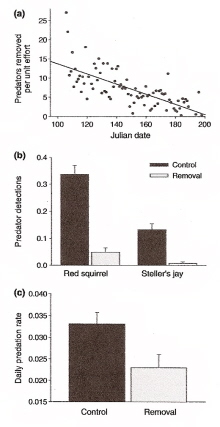 Predator removals resulted in fewer predators present and lower nest predation rates. Capture rates (a) on removal plots during the study and (b) vocalization rates of red squirrels and Steller's Jays, as well as (c) nest predation rates on control and predator-removal plots. |
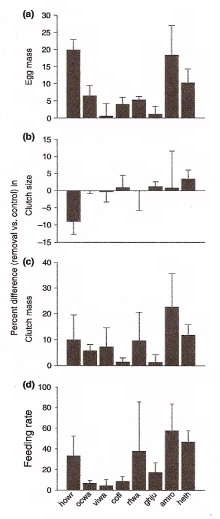 Behavior was altered by predator removal. Responses = % change [(removal - control)/control x 100]. Females in plots with fewer predators (a) laid larger eggs, (b) did not change clutch size, but (c) increased clutch mass. Both parents (d) fed nestlings at higher rates. howr, House Wren; ocwa, Orange-crowned Warbler; viwa, Virginia's Warbler; cofl, Cordilleran Flycatcher; rfwa, Red-faced Warbler; ghju, Gray-headed Junco; amro, American Robin; and heth, Hermit Thrush. |
Nest predator risk and reproductive strategies -- Avian life history theory has long assumed that nest predation plays a minor role in shaping reproductive strategies. Yet, this assumption remains untested by experiments that alter environmental risk of nest predation, despite the fact that nest predation is a major source of reproductive failure. Fontaine and Martin (2006) examined whether parents can assess experimentally reduced nest predation risk and alter their reproductive strategies. They experimentally reduced nest predation risk by removing potential predators from study plots and showed that parents in safer environments increased investment in young through increased egg size, clutch mass, and the rate they fed nestlings. Parents also increased investment in female condition by increasing the rates that males fed incubating females at the nest, and decreasing the time that females spent incubating. These results demonstrate that birds can assess nest predation risk at large and that nest predation plays a key role in the expression of avian reproductive strategies
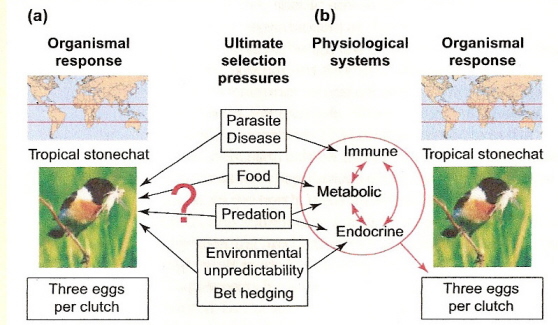
Seasonality, food supply, predation, and pathogens have all been suggested as contributing to the small clutch sizes typical of tropical passerines,
but questions remain concerning the relative importance of these factors and how they might interact (a). Ricklefs and Wikelski (2002) outline a
possible solution to this problem (b). A variety of environmental factors could act on separate physiological systems, each of which could influence
the number of offspring parents can rear. For example, pathogens influence the immune system, food impacts the metabolic system, and environmental
unpredictability might interact with the endocrine system. Because of internal physiological tradeoffs between these systems, and because of system
constraints, the ultimate outcome might be the same: a slow pace of life in tropical passerines, as indicated by a uniformly small clutch size and long life span.
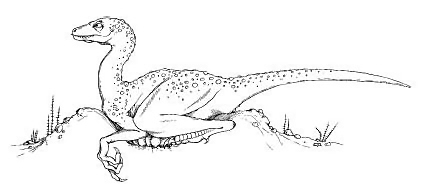
A non-feathered reconstruction of Troodon formosus sitting on its clutch of partially buried, vertically oriented eggs (Horner 2000).
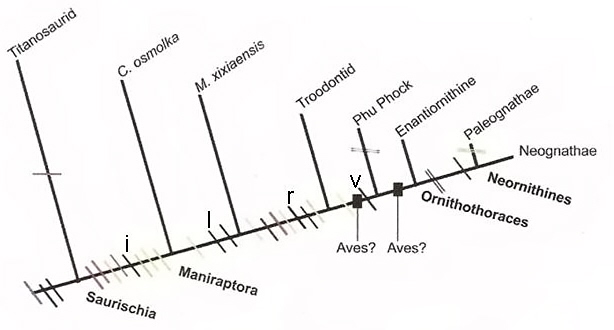
Hypothesized timeline concerning the evolution of eggs and nesting behavior: (i) presence of an air cell in the egg, (l) presence of brooding behavior, (r) reduction from two to one functioning ovary, and (v) presence of proto-avian incubation of eggs. Maniraptors were coelurosaurian dinosaurs, C. osmolka was an oviraptor, Troodontids were small- to medium-sized theropods, Phu Phock refers to where (in Thailand) four small fossil eggs (about the size of goldfinch eggs) from the early Cretaceous were discovered in 2002 and 2003 (Buffetaut et al. 2005). Based on egg-shell characteristics and characteristics of four small bones found at the site, the parent species could have been a primitive bird or a non-avian theropod that exhibited several bird-like characteristics. Enantiornithes is an extinct group of primitive birds from the Mesozoic. Ornithothoraces is a clade of birds that includes all enantiornithines and modern birds (Neornithes) (From: Grellet-Tinner et al. 2006).
|
During incubation, birds transfer heat to eggs. For optimum development, egg temperature must be maintained at about 37 - 38 degrees C (Gill 1995). Exposure to higher temperatures is lethal, while cooler temperatures will, at minimum, slow down or stop development.
Heat is transferred through brood, or incubation, patches - an area of bare, flaccid skin on the abdomen and/or breast. Prior to the initiation of incubation, the skin in the area of the brood patch loses its feathers. In addition, the dermis becomes spongy and richly supplied with blood vessels (Welty and Baptista 1988).
| Birds of nearly all species temporarily shed their feathers on single or paired areas of the breast or abdomen early in the breeding season. The bare skin increases in vascularity, which aids it in transferring body heat for incubating the eggs and brooding the chicks. Development of these incubation (brood) patches is prompted by rising levels of estrogen. They form in whichever sex cares for the eggs and young, usually females but often males as well. The lost feathers are replaced in the complete molt following the breeding season (Stettenheim 2000). | 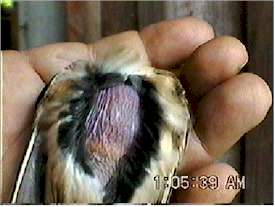 |
Evolution of avian incubation behavior
Incubation is the process by which the temperature of eggs is maintained at levels suitable for embryonic development. During incubation, adult birds not only must transfer heat to eggs, but also maintain an appropriate humidity and turn the eggs on a regular basis. Little is known about the evolution of avian incubation behavior. The discovery of fossilized eggs below the skeleton of an adult oviraptorid suggests that these dinosaurs may have incubated eggs. However, the extent to which body heat was transferred to the eggs is unknown and some investigators believe that oviraptor eggs were completely covered with substrate, probably sand, and one or both adults may have guarded, but not warm, the eggs using direct body contact (Deeming 2002). Although based on circumstantial evidence, other investigators have suggested that troodontids (Troodontidae; a small group of rare and poorly known maniraptorans), with fossils of adults also found on eggs, may have actually warmed (incubated) their eggs. Specifically, troodontids appeared to embed their eggs in the sediment (probably sand) with just a portion exposed (perhaps because reptilian eggs lack a chalaza and cannot be rotated; Burt et al. 2007), and may have incubated eggs sporadically to avoid drastic temperature changes (Grellet-Tinner et al. 2006). Although questions clearly remain, available evidence suggests that caring for and incubating eggs are traits that were likely shared by ancient birds and their immediate ancestors.
Although caring for and incubating eggs likely preceded the origin of birds, less apparent is the question of whether such behavior first involved female-only care, male-only care, or biparental care. Given that most living birds exhibit biparental care, one possible scenario is that biparental care was the ancestral condition for birds. Others have suggested that female-only care is the ancestral condition because, among the few living ectotherms that provide parental care (snakes, crocodilians, snakes and lizards), care is provided almost universally be females, with attendance of unhatched eggs the most common form of parental care (Burley and Johnson 2002). In addition, because females are more certain of maternity than males of paternity and the high degree of anisogamy in birds and their ancestors, female-only care might be more likely to evolve first (Burley and Johnson 2002). Finally, still other authors have described scenarios where male-only care was the ancestral condition, with ancient birds possibly behaving in a manner similar to some living palaeognaths (ratites and tinamous) that exhibit male-only care.
The hypothesis that currently seems most likely is that male-only care is the ancestral condition in birds. Under this scenario (described in more detail by Burt et al. 2007), ectothermic males defended resource-rich territories that attracted females who, after mating with a resident male, deposited eggs in male-prepared depressions in the ground that were then buried in sand or soil. Because the theropods thought by many to be most closely related to birds laid large eggs, ancestral birds may have done so as well. The advantage of larger eggs is that young are larger and more developed at hatching (precocial or even superprecocial) and likely less susceptible to predation. For obvious anatomical reasons, the evolution of larger eggs would likely favor the evolution of sequential laying (one egg in one oviduct at a time), rather than simultaneous (two eggs in two oviducts at a time), laying. Sequential laying would provide females with two other potential benefits: (1) more time to forage and acquire energy and resources for each egg before it was laid, and (2) more opportunities to deposit eggs in the nests of multiple males, a strategy that would increase the likelihood of some eggs hatching (i.e., not all eggs were in one ‘basket’). However, acquiring resources and depositing eggs in multiple nests would make it impossible for females to guard all their eggs or nests. Although this may be a reasonable scenario from the female perspective, what selective pressures would favor male care of nests and, perhaps, young?
The evolution of male-only care of nests would, of course, require that males receive some fitness benefit. One possible benefit is that males defending territories and guarding nests might still be able to court and copulate with additional females. In fact, females might have preferred males whose nests already contained eggs because of the dilution effect (an individual egg in a large clutch is less likely to be predated than one in a smaller clutch). Among early endothermic species, males may have benefited even more by covering, or incubating, buried eggs in shallow nests or partially buried eggs (as in the nests of Troodon formosus) because the application of heat, particularly at night, would increase rates of embryonic development, shorten the ‘incubation’ period, and reduce the chances of nest predation.
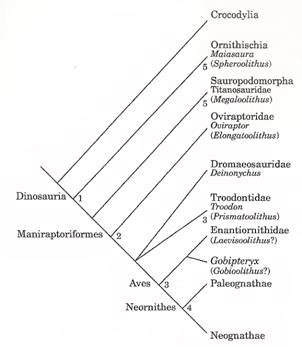
Archosaurian phylogeny showing relationship between Troodontids (Troodontidae)
and Aves (Zelenitsky et al. 2002).
Evidence to date supports the hypothesis that male-only care was likely the ancestral condition for birds. To assess parental care in Cretaceous troodontid and oviraptorid dinosaurs, Varricchio et al. (2008) examined clutch volume (total volume of all eggs in a clutch) and the bone histology of brooding adults. These authors found that the relatively large clutch volumes of Troodon, Oviraptor, and Citipati were similar to those of living paleognaths with polygamous mating systems and extensive male care, including Ostriches, Emus, and Rheas. Clutch volumes can evolve to be larger in species without maternal care because females may have more resources to devote to eggs if they provide no care and because a clutch may be composed of eggs from multiple females. In addition, the fossil bones of adult troodontids and oviraptorids found associated with nests did not have histologic features common to those of female archosaurs (including birds) in breeding condition. That is, female archosaurs extract substantial amounts of calcium and phosphorus from their skeletal tissues during egg formation. Histologic examination of cross sections of bones (femur, tibia, and a metatarsal bone) from an adult Troodon found in direct contact with an egg clutch revealed little evidence of bone remodeling or bone resorption, suggesting that the bones were those of a male.

A male of the medium-sized predatory dinosaur Troodon (that lived in North America in the late Cretaceous Period) brooding a clutch
of eggs. Fossilized remains of Troodon and two other types of dinosaurs found with large clutches of eggs suggest that males, and not females,
protected
and incubated eggs laid by perhaps several females (Credit: Bill Parsons)
Paternal care in both troodontids and oviraptorids indicates that this care system evolved before the emergence of birds and represents the ancestral condition for birds. If so, the biparental care that is so prevalent among living birds represents a derived condition. Although most current evidence seems to support the hypothesis that male-only care was the ancestral condition for birds, additional study, and the discovery of new fossils, may still shed additional light on this question.
 |
Incubation reduces microbial growth on eggshells -- Avian eggshells harbor microbes shortly after laying, and under appropriate ambient conditions they can multiply rapidly, penetrate through shell pores, infect egg contents and cause embryo mortality. Cook et al. (2005) experimentally examined how incubation affects bacterial processes on the eggshells of Pearl-eyed Thrashers (Margarops fuscatus) nesting in tropical montane and lowland forests in Puerto Rico. Bacteria and fungi grew rapidly on shells of newly laid, unincubated eggs exposed to ambient conditions, but declined to low levels on shells of eggs incubated by thrashers. Divergence in bacterial growth between incubated and exposed eggs was more marked at the montane forest than at the lowland site. Pathogenic microorganisms became increasingly dominant on shells of exposed eggs, but these groups were relatively rare on incubated eggs, where more benign, less invasive groups prevailed. Some incubation during laying may be necessary to decrease the probability of trans-shell infection by reducing the growth of harmful bacteria and fungi on eggshells, although it may increase hatching asynchrony and the likelihood of brood reduction. |
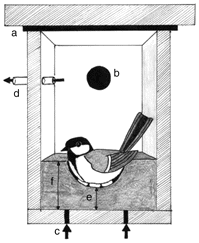
A nest-box modified into a metabolic chamber. To ensure the top of the nest-box was airtight, a sheet of rubber was inserted between nest-box and lid (a) and a cork was placed in the entrance hole (b). Reference air was measured close to the inflow of the nest-box (arrows underneath nest-box; c), while sample air was drawn from the nest-box via a tube near the entrance hole (d). The thickness of the nest was determined by the thickness of the nest cup (e) and the height of the nest rim (f).
Energetic cost of incubation -- In birds, the annual peak of energy demand has long been thought to occur when parents provision their offspring with food during the nestling phase. This has lead to the idea that selection on clutch size takes place during the nestling phase. As a result, the energetic demands during other reproductive phases – such as egg laying and incubation – have long been ignored. During incubation, avian eggs need external heat provisioning, regular turning and favourable humidity for proper embryonic development; care that is often provided by one or both of the parents. The energetic costs of providing heat to the eggs have long been thought to be negligible. Increasing evidence suggests, however, that below thermo-neutrality the metabolic rate (energy spent per time unit) of an incubating female is higher than that of a nonincubating female at rest. Because temperatures are normally below thermoneutrality at temperate latitudes, energetic costs of incubation may substantially add to the overall daily energy expenditure of attending parents.
de Heij et al. (2007) manipulated the clutch sizes of female Great Tits (Parus major) and monitored their metabolic rates during nocturnal incubation using mobile oxygen analyzers. They found that clutch enlargement caused incubating females to expend more energy, but clutch reduction did not result in a lowering of energy expenditure.The absence of an effect of clutch reduction can likely be explained by a limit to the number of eggs that can be in direct contact with the brood patch, i.e., the `threshold clutch size'. Above such a threshold clutch size, eggs that are not in contact with the female's brood patch likely cool. Consequently, incubating birds will repeatedly rearrange the eggs to rewarm the cooled eggs. Rewarming has been shown to be energetically more costly than maintaining eggs at incubation temperatures. Thus, energetic costs increase when clutch size is above the threshold clutch size, but do not change when clutch size is at or below threshold clutch size.
de Heij et al. (2007) also found that incubating birds with thicker nests had lower energy expenditure, probably because thicker nests were better insulated. The fact that not all birds build well-insulated nests suggests that there is a cost to thick nests. Knowing that females expend more energy during nocturnal incubation when incubating experimentally enlarged clutches is a first step towards determining a potential mechanism underlying negative selection on clutch size during the incubation phase. However, measurements on energy expenditure over a full 24 h are needed to judge how important energy expenditure can be in explaining fitness consequences of incubating experimentally enlarged clutches.
Most birds sit on their eggs to incubate, but there are exceptions. For example, male Emperor and King penguins place their egg on their feet, wrap their wing around the egg, and incubate it while standing up. The egg is kept warm by the heat from the male's feet & wing. Megapodes, found in Southeast Asia and Australia, use heat sources such as geothermal, solar, or decomposition of organic material.
Male Emperor Penguins
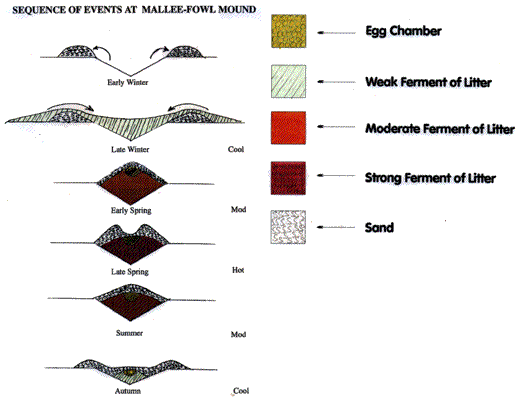
Malleefowl (Leipoa ocellata) dedicate 9-11 months
per year building and maintaining a large incubation mound of soil, leaves
and
twigs. The eggs are laid in the mound, buried and left
to incubate by heat generated by the composting litter. Malleefowl
mounds may be used over many generations and can attain
an impressive size of 22 meters in circumference and one
meter high. The birds attempt to maintain the mound temperature
of 32-34 degrees C by using their beak as a "thermometer" and
adjusting soil cover to either retain or expel
heat from the egg chamber. 'Incubation' typically takes 60 - 90 days.
(Source: http://www.malleefowl.com.au/Pages/TheMalleefowl.htm)
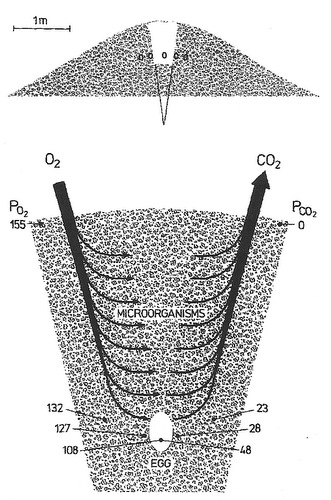
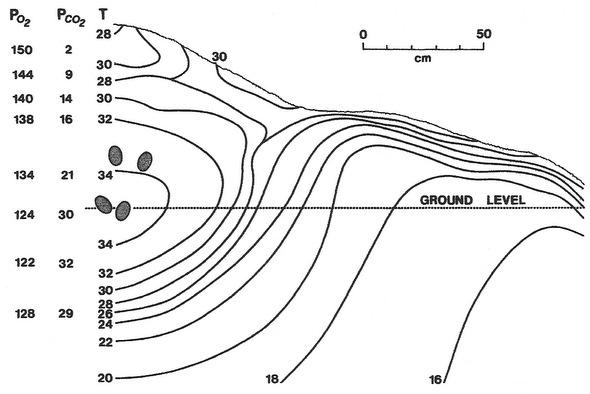 Incubation mound of a Mallee Fowl (Leipoa ocellata), above, and cross-section through a typical Australian Brush-Turkey (Alectura lathami) mound, right. Levels of oxygen and carbon dioxide in mounds are influenced by diffusion and, importantly, by the respiration of microorganisms. In the diagram of the Brush Turkey mound (right), numbers represent partial pressures of oxygen and carbon dioxide at the mound surface, at the level of the egg, and outside and inside the egg. Due to the respiration of microorganisms, oxygen levels around eggs are lower and carbon dioxide levels around eggs are higher than atmospheric levels. This results in a smaller diffusion gradient between the developing embryo and its external environment than is the case for other birds where eggs are not buried, potentially making the exchange of gases less efficient. However, eggs of Mallee Fowl and Brush Turkeys (and other megapodes) have thinner shells than other birds of comparable size, allowing more efficient conductance of gases (Source of figures: Seymour and Ackerman 1980, Seymour et al. 1986). |
 |
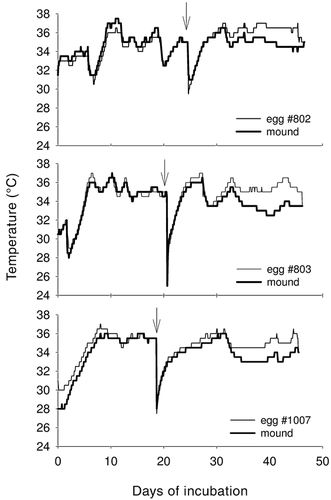
Male Australian Brush-Turkeys (Alectura lathami) attempt to minimize the amount of work needed to maintain the appropriate temperature (about 34°C) by tossing litter onto mounds so it is not compacted and using dryer litter to keep water content low (Seymour and Bradford 1992). Low water content is important because wet mounds have higher thermal conductance and lose heat more rapidly. Males typically visit mounds daily and spend 0.5 to 2 hours working on the mounds (Eiby and Booth 2008), periodically digging down to the eggs, testing the temperature with their head or bill, then opening or closing the mound and adding or removing vegetation as needed. Although average mound temperatures are generally close to 34°C, temperatures do fluctuate, especially when it rains (Figure below). In three closely monitored mounds, mean temperatures were 33.8°C, but daily temperatures ranged from 24.5 to 37.5°C (Eiby and Booth 2008). However, in contrast to the embryos in most species of birds, brush-turkey embryos are able to tolerate such temperature fluctuations and develop normally.
Figure to the right. Temperature of eggshell surfaces and mound material adjacent to eggs of Australian Brush-Turkeys from the time of laying (day 0) until hatching in three mounds. All mounds experienced fluctuations in temperature, especially on and after days with heavy rain (indicated by the arrows). Egg temperatures increased toward the end of incubation because of metabolic heat production by embryos. Eggshell temperatures falling to match mound temperatures on the right indicate hatching (From: Eiby and Booth 2008). |
 |
| Foot-Mediated Incubation: Nazca Booby (Sula granti) Feet as Surrogate Brood Patches -- Incubation in most avian species involves transferring heat from parent to egg through a highly vascularized brood patch. Some birds, however, do not develop a brood patch. Unusual among birds, these species hold their eggs under the webs of their feet, but the role of the feet in heat transfer is uncertain. Often the webs are positioned between the feathered abdomen and the egg during incubation, suggesting that either the abdomen, the feet, or both could transfer heat to the egg. Morgan et al. (2003) studied heat transfer from foot webs to eggs during incubation in Nazca boobies by spatially separating the feet from the abdomen using an oversized egg. They found that feet transfer heat to eggs independently of any heat that may be transferred from the abdomen. In addition, they found that incubating boobies had significantly greater vascularization in their foot webs, measured as a percentage of web area covered by vessels, than nonincubating boobies. In addition, males, whether incubating or nonincubating, had significantly less web vascularization than females. These results indicate that vascularized Nazca booby feet function in the same way during incubation that vascularized brood patches do, acting as surrogate brood patches. |
Incubation periods range from about 10 days for some passerines & woodpeckers to as many as 80 days for albatross' and kiwis. The time spent incubating can be influence by the size of the egg, the state of development at hatching (precocial vs. altricial), & ambient temperature.
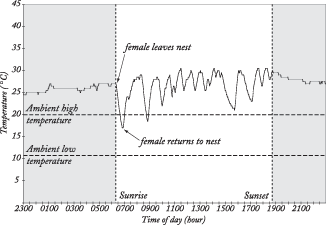
Temperatures in an Eastern Bluebird nest cup in Texas
(Cooper
and Phillips 2002).
However, much of the variation in the duration of incubation periods is due to phylogeny, with genetic factors that control embryonic growth rates playing a significant role in determining how long any incubation period needs to be for any particular order of birds. This phylogenetic effect is apparent when comparing relationships between egg mass and incubation period for different orders of birds. For example, among grebes (Podicipediformes) egg mass for different species varies more the four-fold ( 10 – 42 gms), but incubation periods vary only by 10 days (18 – 28 days). Similarly, among gallinaceous birds (Galliformes) egg mass varies more than 12-fold (8 – 102 gms), but incubation periods vary by only 11 days (18 – 29 days). About 80% of the variation in incubation periods among birds is due to differences between orders and between families within orders; only about 20% of the variation is due to differences between genera, species, and populations (Ricklefs and Starck 1998, Bennett and Owens 2002).
| Nest predation appears to affect the evolution of parental behavior -- Based on an analysis of the incubation and provisioning behavior of 97 species of passerines, Conway and Martin (2000) suggested that environments with a high risk of nest predation may favor long on-bouts (long periods on the nest) and few foraging trips. However, such an incubation strategy may prevent frequent feeding by adults and thus compromise future reproductive attempts. Therefore, nest predation may influence the evolution of avian life-history traits in several ways. High nest predation favors a bet-hedging strategy of holding back reproductive effort for renesting attempts and survival, a short nesting cycle to minimize the time nests are susceptible to predation, and small brood size to minimize noise of begging young Yet, Conway and Martin's (2000) results suggest that nest predation may influence passerine life-history evolution in a way that has been largely ignored by placing constraints on parental activity and the way an incubating female allocates her time between incubation and foraging. Thus, in environments with high nest predation, natural selection simultaneously favors infrequent nest trips (to reduce the probability of predator detection) and short off-bout duration (to maximize development rates and reduce time of exposure to predators). These somewhat opposing constraints limit the range of effective incubation strategies available to females in environments with high nest predation. |
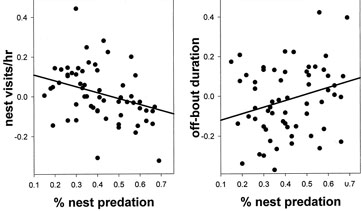
Scatter plot of nest predation versus unstandardized residuals (partial regressions) of incubation behaviors corrected for diet, foraging strategy, nest substrate, body mass, mate feeding frequency, and temperature. Conway & Martin (2000) arcsine-transformed nest predation and log-transformed body mass and temperature prior to calculating residuals. Lines represent slopes of significant linear regressions |
Camouflage
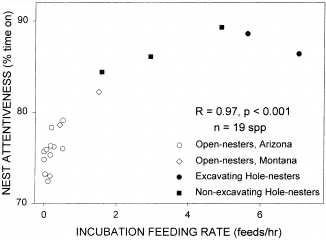 Nest attentiveness (percentage of time that the female is incubating on the nest) relative to the rate that males bring food to the nest (incubation feeding). The relationship across 19 species of both open- and cavity-nesters is curvilinear and significant. Diamonds represent species studied in Montana, and circles and squares represent species studied in Arizona. Open symbols represent open nesters, and solid symbols represent hole nesters, where solid squares are nonexcavators and solid circles are excavators (From: Martin and Ghalambor 1999). |
Males Feeding Females during Incubation -- Nest attentiveness (percentage of time spent on the nest) during incubation represents a parent-offspring conflict; incubating birds must balance a trade-off between caring for embryos by staying on the nest versus caring for themselves by getting off the nest to forage. For species in which females are the sole incubator, males can potentially affect this trade-off and increase nest attentiveness by feeding incubating females on the nest (incubation feeding). Increased nest attentiveness may be required when local microclimate conditions are harsh and thereby require greater incubation feeding (microclimate hypothesis). Alternatively, incubation feeding may be constrained by risk of attracting nest predators (nest predation hypothesis), which in turn may constrain female nest attentiveness because of energy limitation. We show that incubation feeding rates are much greater among cavity-nesting than among coexisting open-nesting birds. Under the microclimate hypothesis, the greater incubation feeding rates of cavity-nesting birds generate the prediction that microclimate should be harsher than for open-nesting birds. Our results reject this hypothesis because we found the opposite pattern; cavity-nesting birds experienced more moderate (less variable) microclimates that were less often below temperatures (i.e., 16°C) that can negatively impact eggs compared with open-nesting species. In contrast, incubation feeding rates were highly negatively correlated with nest predation both within and between the two nest types, supporting the nest predation hypothesis. Incubation feeding in turn was positively correlated with nest attentiveness. Thus, nest predation may indirectly affect female incubation behavior by directly affecting incubation feeding by the male (Martin and Ghalambor 1999). (Check this short video of a male Blue Tit feeding his mate). |
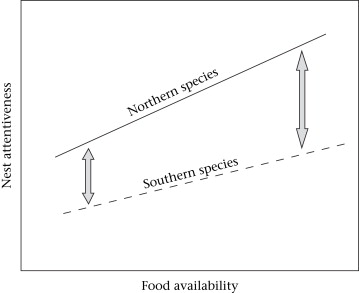
Hypothesized relationship of food availability and avian nest attentiveness across species and latitudes.
Both northern and southern species are expected to show proximate increases in attentiveness in response to increased food availability,
and preliminary data further suggest that northern species show slightly stronger responses. Proximate responses to food availability alone, however,
cannot explain why southern species generally show lower nest attentiveness than similar northern species (grey arrows).
Latitudinal variation in avian incubation attentiveness -- Avian incubation attentiveness has important fitness consequences through its influence on the number and quality of hatched young and energetic costs imposed on parents. Nest attentiveness is highly variable across species and geographical regions. Chalfoun and Martin (2007) reviewed the literature and found a worldwide pattern that nest attentiveness of passerines is generally lower in south temperate and tropical regions than in north temperate regions. They also conducted a food manipulation experiment to assess the extent to which nest attentiveness may reflect proximate responses versus an evolved behaviour. Chalfoun and Martin (2007) used the Karoo Prinia (Prinia maculosa) in South Africa, which has very low nest attentiveness (about 49%) compared with that of many passerine birds. They provided supplemental food during early incubation to experimental females and compared nest attentiveness and on- and off-bout lengths of experimental and paired control females. Nest attentiveness of females at food-provisioned nests was significantly higher than that of control females (57% vs. 49%). Food-supplemented females also spent significantly less time off the nest than did control females, whereas mean on-bout lengths did not differ. However, mean nest attentiveness of food-provisioned females was still substantially below that of other similar bird species worldwide. Food can be an important proximate influence on parental care behaviour, but proximate influences of food do not explain broad latitudinal patterns of attentiveness. Climatic variation across latitudes may influence the amount of time that parents spend on the nest, although temperatures at many south temperate sites often approximate those at north temperate sites during the breeding season. One possible alternative explanation for geographical patterns in nest attentiveness is variation in adult mortality across latitudes. According to classic life history theory, if southern birds experience lower adult mortality, they should be less willing to invest as much in nest attentiveness and other components of current reproduction. Testing for the existence of an adult mortality–nest attentiveness trade-off across latitudes is therefore a critical next step in addressing geographical variation in parental care strategies.
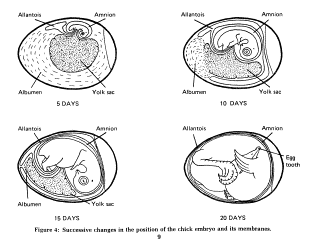
Development of the Embryo (Check this site: http://ag.ansc.purdue.edu/poultry/clipart.htm & scroll down to 'chicken embryos')
|
|
 |
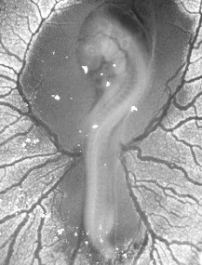 |
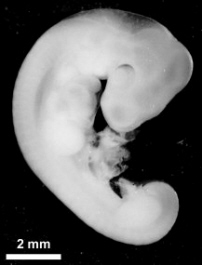 |
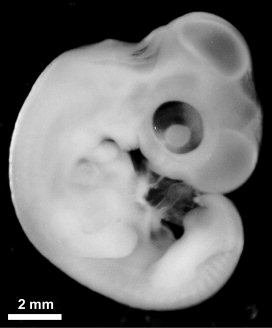 |
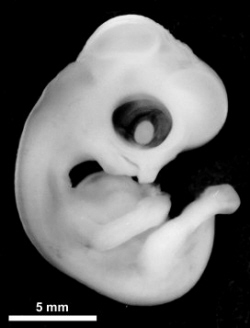 |
 |
 |
 |
Source: http://www.agaction.com/Resources/Animalbook/avianembryo.htm
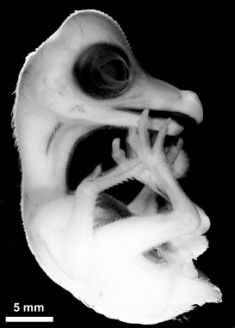
Four-day-old chicken embryo
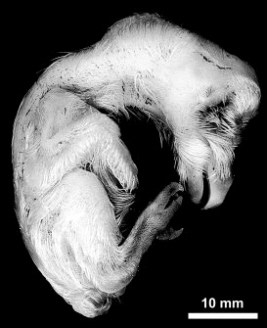
16-day-old chicken embryo
Many birds have incubation periods much shorter than 21 days (e.g., many songbirds have incubation periods of 9 - 11 days). In those species, the 'events' listed above would, of course, occur much earlier in incubation.
American Robin nestlings
During development in an egg, yolk and albumen are being converted into an embryo, with yolk (and, specifically, lipids or fats in the yolk) providing energy and albumen providing protein and water. This conversion process involves both differentiation and growth. Differentiation of avian embryos involves the development of a three-dimensional structure from a flat plate of cells (blastoderm) on top of the yolk. The center of the blastoderm will become the embryo and peripheral cells give rise to the yolk sac and amnion. During a process called gastrulation, the development embryo in rearranged into three layers: endoderm, mesoderm, and ectoderm. The endoderm will ultimately form the nervous system, skin, and parts of the eye, the mesoderm becomes skeletal muscles, the skeleton, and internal structures, such as the circulatory and excretory systems, and the endoderm forms the digestive tract, respiratory passages, and organs such as the liver and pancreas.
The faster-growing embryos of altricial birds initially allocate more energy to the rapid development of tissues and organs, such as those of the digestive system, that supply the nutritional demands of other tissues and organs (Karlsson and Lilja 2008), and less energy to development of organs and structures such as the brain, muscles, skeleton, and feathers (Blom and Lilja 2005). As a result, altricial embryos typically hatch with smaller brains, immature skeletal muscles, and poorly ossified skeletons, whereas precocial embryos hatch with larger brains, mature muscles, and highly ossified skeletons (Figure below). One consequence of these differences, particularly the greater ossification of the skeleton, is that precocial embryos have a greater need for calcium during development. Some of this calcium comes from the yolk, but most (about 80%) comes from the eggshell. As might be expected, the eggshells of precocial species contain more calcium than those of altricial species (Karlsson and Lilja 2008).
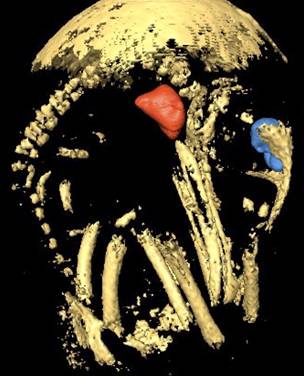
A CAT scan image of a 17-day-old Domestic Chicken embryo in an intact egg. Part of the egg shell can be seen at the top of the image.
Several well-ossified bones can be seen as well as the lungs (red) and brain (blue) (From: Sutendra and Michelakis 2007).
Metabolic rates of avian embryos
Embryos in species with different modes of development exhibit differences in metabolic rates. In precocial species, metabolic rates (as measured by oxygen consumption; T in Figure below) increases rapidly during most of the incubation period, but then typically level off or even decline when incubation is about 80% completed. In altricial species, on the other hand, metabolic rates of embryos increase continuously throughout the entire incubation period (Figure below). In altricial birds, embryo mass and growth rates (grams of new tissue added per day; G in Figure below) typically increase continuously throughout incubation and, as a result, the energetic costs of maintenance and synthesizing new tissue increase until hatching. However, in precocial species, embryo mass approaches the typical mass at hatching when incubation periods are about 80% complete. As a result, the amount of energy devoted to growth either levels off or declines toward the end of incubation. Maintenance costs continue to increase, but the reduced growth causes a leveling off or reduction in the total metabolic rate (Figure below).
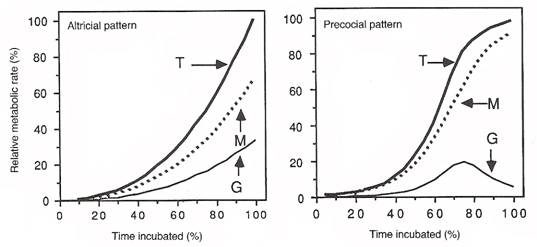
Comparison of embryo metabolic rates of altricial and precocial birds. Energy devoted to growth (G) and maintenance (M) added
together is equal to the total metabolic rate (T). (From: Vleck and Hoyt 1991).
Despite differences in metabolic rates during incubation, the embryos of altricial and precocial species do not appear to differ in the amount of energy needed to generate new tissue. Rather, the difference between altricial and precocial embryos lies in the timing of hatching during the developmental process (earlier for altricial species than for precocial species) and in the amount of energy females deposit in eggs. Because of the relatively shorter incubation periods and because embryos hatch at an earlier stage of development, females in altricial species can invest less energy (less yolk) in eggs than females in precocial species (Vleck and Vleck 1987).

Photo by Alexander Badyaev |
Mother Finches Control Baby's Sex -- Most mothers-to-be simply hope for healthy offspring. But female House Finches tip the odds in their babies' favor by pre-determining their gender. Female House Finches (Carpodacus mexicanus) adjust the sex and growth of their offspring to account for the order in which the eggs are laid, thereby reducing mortality of their sons and daughters by 10 - 20%. Badyaev et al. (2002) studied two populations of House Finches that have diverged significantly over the past 20 years. They found patterns in how a baby finch's sex and position in the laying order affected both its growth pattern and chance of survival. In Montana, first-born females exhibited better survival odds than males. But in Alabama, first-born males survived more often. Such differences seem to drive maternal finches to select whether sons or daughters hatch first. "Breeding females in both Montana and Alabama populations lay male and female eggs in different sequences within clutches," the authors write, "thus placing sons and daughters in the most advantageous positions for survival in that particular environment." Exactly how finch mothers control offspring sex, survival and growth remains a mystery, but such adjustments facilitate adaptation to local environments. Observing such acclimatization provides "empirical support for the hypothesis that parental effects play a crucial role at the initial stages of population divergence by enabling establishment of populations in novel environments." -- Sarah Graham, Scientific American |
About three days before hatching,
the embryo's head burrows beneath the right shoulder so the beak is positioned
under the wing & against the two membranes separating the embryo from
the air space at the large end of the shell. Sometime that same  day,
the beak pierces through the membranes into the air space & pulmonary
respiration begins. About a day later, with a dwindling oxygen supply,
the embryo begins to kick, to twist and to thrust its head and beak backward,
until the egg
tooth pips the first hole. The chick can now draw breath. As fresh
air enters the shell and circulates, the membranes inside begin to dry,
and the blood vessels within those membranes begin to shrink. The embryo
continues to pip, kick and twist. Small cracks advance counter-clockwise
by millimeters around the big end of the shell. A special "hatching muscle"
on the back of the chick's neck (see photo to the right) swells to several
times its normal size with a great influx of fluid from the embryo's lymphatic
system. This swelling accentuates sensory signals sent through the neck,
stimulating the embryo to further activity. Eventually, the cap of the
egg is cracked enough. The embryo pushes it off, unfolds from the tuck,
and escapes from the shell. (See Budgerigars
hatching)
day,
the beak pierces through the membranes into the air space & pulmonary
respiration begins. About a day later, with a dwindling oxygen supply,
the embryo begins to kick, to twist and to thrust its head and beak backward,
until the egg
tooth pips the first hole. The chick can now draw breath. As fresh
air enters the shell and circulates, the membranes inside begin to dry,
and the blood vessels within those membranes begin to shrink. The embryo
continues to pip, kick and twist. Small cracks advance counter-clockwise
by millimeters around the big end of the shell. A special "hatching muscle"
on the back of the chick's neck (see photo to the right) swells to several
times its normal size with a great influx of fluid from the embryo's lymphatic
system. This swelling accentuates sensory signals sent through the neck,
stimulating the embryo to further activity. Eventually, the cap of the
egg is cracked enough. The embryo pushes it off, unfolds from the tuck,
and escapes from the shell. (See Budgerigars
hatching)

Source:
http://www.nwf.org/internationalwildlife/eggs.html
Canada Goose hatching
Dinosaur embryos tucked inside eggs just like birds
Winter
habitat influences reproductive success --
The destruction of tropical forests is creating serious breeding problems
for migratory birds. Norris et al. (2004) found that the quality of winter
habitat affected the ability of American Redstarts to successfully reproduce
when they returned north in the spring. "Our findings help explain why
many species of long-distance migratory songbirds have declined over the
last few decades," says Mr. Norris, noting that a relatively small geographic band – across the Caribbean,
Greater Antilles, and central America – is the annual destination of an
estimated five billion migratory birds flying south each year from Canada.
Norris et al. (2004) measured stable carbon isotopes in blood samples collected
from American Redstarts after the birds arrived at their breeding grounds
in Ontario. Since the turnover time of the blood cells is from six to eight
weeks, they provide a good indicator of the quality of the birds' previous
habitat on the wintering ground. The carbon signature of each redstart
has been deposited by insects the bird ate, and the insects in turn fed
on vegetation growing in the winter habitat. "It's basically a 'food chain
signature'," says Mr. Norris. The researchers first determined what were
high and low quality habitats for redstarts, which winter in the Caribbean
and central America, and breed in deciduous forest throughout Canada and
the U.S. They found that what makes a better winter habitat is primarily
the degree of wetness, particularly at the end of the season (late winter/early
spring) when low quality habitats tend to dry out. The second step was
to develop isotope "markers" that identify the quality of each type of
habitat. Next, blood samples were taken from warblers in their breeding
grounds, and finally the birds' reproductive success was measured by counting
the number of "fledged" offspring to leave their nests. Analysis revealed
that redstarts wintering in high quality habitats, such as mangroves and
lowland tropical forests, arrived earlier on the breeding grounds, nested
earlier, and were more successful in producing young. This study shows
that destroying high quality winter habitat has a disproportionate effect
on the redstart populations: they lose the areas most capable of supporting
them. noting that a relatively small geographic band – across the Caribbean,
Greater Antilles, and central America – is the annual destination of an
estimated five billion migratory birds flying south each year from Canada.
Norris et al. (2004) measured stable carbon isotopes in blood samples collected
from American Redstarts after the birds arrived at their breeding grounds
in Ontario. Since the turnover time of the blood cells is from six to eight
weeks, they provide a good indicator of the quality of the birds' previous
habitat on the wintering ground. The carbon signature of each redstart
has been deposited by insects the bird ate, and the insects in turn fed
on vegetation growing in the winter habitat. "It's basically a 'food chain
signature'," says Mr. Norris. The researchers first determined what were
high and low quality habitats for redstarts, which winter in the Caribbean
and central America, and breed in deciduous forest throughout Canada and
the U.S. They found that what makes a better winter habitat is primarily
the degree of wetness, particularly at the end of the season (late winter/early
spring) when low quality habitats tend to dry out. The second step was
to develop isotope "markers" that identify the quality of each type of
habitat. Next, blood samples were taken from warblers in their breeding
grounds, and finally the birds' reproductive success was measured by counting
the number of "fledged" offspring to leave their nests. Analysis revealed
that redstarts wintering in high quality habitats, such as mangroves and
lowland tropical forests, arrived earlier on the breeding grounds, nested
earlier, and were more successful in producing young. This study shows
that destroying high quality winter habitat has a disproportionate effect
on the redstart populations: they lose the areas most capable of supporting
them. |
Literature Cited:
Badyaev, A.V., G. E. Hill, M.L. Beck, A.A. Dervan, R.A. Duckworth, K.J. McGraw, P.M. Nolan, and L.A. Whittingham 2002. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316-318.
Bell, C. P. 1996. The relationship between geographic variation in clutch size and migration pattern in the Yellow Wagtail. Bird Study 43: 333-341.
Bennett, P. M., and I. P. F. Owens. 2002. Evolutionary ecology of birds: life histories, mating systems and extinction. Oxford University Press, Oxford, UK.
Blom, J., and C. Lilja. 2005. A comparative study of embryonic development of some bird species with different patterns of postnatal growth. Zoology 108: 81-95.
Böhning-Gaese, K., B. Halbe, N. Lemoine, and R. Oberrath. 2000. Factors influencing the clutch size, number of broods and annual fecundity of North American and European land birds. Evolutionary Ecology Research 2: 823-839.
Buffetaut, E., G. Grellet-Tinner, V. Suteethorn, G. Cuny, H. Tong, A. Kosir, L. Cavin, S. Chitsing, P. J. Griffiths, J. Tabouelle, and J. Le Loeuff. 2005. Minute theropod eggs and embryo from the Early Cretaceous of Thailand and the dinosaur-bird transition. Naturwissenschaften 92: 477-482.
Burley, N. T., and K. Johnson. 2002. The evolution of avian parental care. Philosophical Transactions of the Royal Society B 357: 241-250.
Burt, D. B., P. F. Coulter, and J. D. Ligon. 2007. Evolution of parental care and cooperative breeding. In: Reproductive biology and phylogeny of birds, part B (B. G. M. Jamieson, ed.), pp. 295-325. Science Publishers, Enfield, NH.
Chalfoun, A. D. and T. E. Martin. 2007. Latitudinal variation in avian incubation attentiveness and a test of the food limitation hypothesis. Animal Behaviour 73: 579-585.
Conway, C. J. and T. E. Martin. 2000. Evolution of passerine incubation behavior: influence of food, temperature, and nest predation. Evolution 54: 670-685.
Cook, M. I., S. R. Beissinger, G. A. Toranzos, and W. J. Arendt. 2005. Incubation reduces microbial growth on eggshells and the opportunity for trans-shell infection. Ecology Letters 8:532-537.
Cooper, C. and T. Phillips. 2002. Rhythm and Bluebirds. Birdscope, newsletter of the Cornell Lab of Ornithology, Summer 2002. <www.birds.cornell.edu>
Deeming, D. C. 2002. Importance and evolution of incubation in avian reproduction. In: Avian incubation (D. C. Deeming, ed.), pp. 1-7. Oxford University Press, Oxford, UK.
de Heij, M. E., A. J. van der Graaf, D. Hafner, and J. M. Tinbergen. 2007. Metabolic rate of nocturnal incubation in female Great Tits, Parus major, in relation to clutch size measured in a natural environment. Journal of Experimental Biology 210: 2006-2012.
Eggers, S., M. Griesser, M. Nystrand and J. Ekman. 2006. Predation risk induces changes in nest-site selection and clutch size in the Siberian Jay. Proceedings of The Royal Society B 273:701-706.
Eiby, Y., and D. Booth. 2008. Embryonic thermal tolerance and temperature variation in mounds of the Australian Brush-Turkey (Alectura lathami). Auk 125: 594-599.
Fontaine, J. J. and T. E. Martin. 2006. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecology Letters 9: 428-434.
Gill, F.B. 1995. Ornithology, second ed. W.H. Freeman and Co., New York.
Grellet-Tinner, G., L. Chiappe, M. Norell, and D. Bottjer. 2006. Dinosaur eggs and nesting behaviors: a paleobiological investigation. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 294-321.
Horner, J. R. 2000. Dinosaur reproduction and parenting. Annual Review of the Earth and Planetary Sciences 28:19–45.
Jetz, W., C. H. Sekercioglu, and K. Böhning-Gaese. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biology 6: e303.
Karlsson, O., and C. Lilja. 2008. Eggshell structure, mode of development and growth rate in birds. Zoology 111: 494-502.
Köppl, C., E. Futterer, B. Nieder, R. Sistermann, and H. Wagner. 2005. Embryonic and posthatching development of the Barn Owl (Tyto alba): reference data for age determination. Developmental Dynamics 233: 1248-1260.
Lack, D. 1947. The significance of clutch size. Ibis 89: 302-352.
Martin, T. E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecological Monographs 65: 101-127.
Martin, T. E. and C. K. Ghalambor. 1999. Males feeding females during incubation. I. Required by microclimate or constrained by nest predation? American Naturalist 153: 131-139.
Martin, T.E., P. R. Martin, C. R. Olson, B. J. Heidinger, & J. J. Fontaine. 2000. Parental care and clutch sizes in North and South American birds. Science 287: 1482-1485.
Martin, T. E., J. Scott, and C. Menge. 2000. Nest predation increases with parental activity: separating nest site and parental activity effects. Proceedings of the Royal Society B 267: 2287-2293.
Mauck, R. A., C.E. Huntington, and T.C. Grubb. 2004. Age-specific reproductive success: evidence for the selection hypothesis. Evolution 58: 880-885.
Morgan, S. M., M. A. Ashley-Ross, and D. J. Anderson. 2003. Foot-mediated incubation: Nazca Booby (Sula granti) feet as surrogate brood patches. Physiological and Biochemical Zoology 76: 360.
Nager, R., P. Monaghan, & D. C. Houston. 2001. The cost of egg production: increased egg production reduces future fitness in gulls. Journal of Avian Biology 32:159-166.
Norris, D.R., P. P. Marra, T. K. Kyser, T. W. Sherry, and L.M. Ratcliffe. 2004. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. R. Soc. B 271:59-64.
Ricklefs, R. E. 1997. Comparative demography of New World populations of thrushes (Turdus spp.). Ecological Monographs 67: 23-43.
Ricklefs, R. E. 2000. Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor 102: 9-22.
Ricklefs, R. E., and J. M. Starck. 1998. Embryonic growth and development. In: Avian growth and development (J. M. Starck and R. E. Ricklefs, eds.), pp. 31-58. Oxford University Press, Oxford, UK.
Ricklefs, R. E. and M. Wikelski. 2002. The physiology/life-history nexus. Trends in Ecology and Evolution 17: 462-468.
Rohrbaugh, R. 1998. Citizen science hits the big
time in St. Louis. Birdscope 12:3-4.
Rowe, L., D. Ludwig, and D. Schluter. 1994. Time, condition, and the seasonal decline of avian clutch size. American Naturalist 143: 698-722.
Seymour, R. S., and R. A. Ackerman. 1980. Adaptations to underground nesting in birds and reptiles. American Zoologist 20: 437-447.
Seymour, R. S., and D. F. Bradford. 1992. Temperature regulation in the incubation mounds of the Australian Brush-Turkey. Condor 94: 134-150.
Seymour, R. S., D. Vleck, and C. M. Vleck. 1986. Gas exchange in the incubation mounds of megapode birds. Journal of Comparative Physiology B 156: 773-782.
Slagsvold, T. 1982. Clutch size variation in passerine birds: the nest predation hypothesis. Oecologia 54: 159-169.
Sockman, K. W., P. J. Sharp, and H. Schwabl. 2006. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biological Reviews 81: 629-666.
Stettenheim, Peter R. 2000. The Integumentary Morphology of Modern Birds—An Overview. American Zoologist 40: 461-477.
Stutchbury, B. J. M., and E. S. Morton. 2008. Recent advances in the behavioral ecology of tropical birds. Wilson Journal of Ornithology 120: 26-37.
Sutendra, G., and E. D. Michelakis. 2007. The chicken embryo as a model for ductus arteriosus developmental biology: cracking into new territory. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 292: R481-R484.
Varricchio, D. J., J. R. Moore, G. M. Erickson, M. A. Norell, F. D. Jackson, and J. J. Borkowski. 2008. Avian parental care had dinosaur origin. Science 322: 1826-1828.
Vleck, C. M., and D. F. Hoyt. 1991. Metabolism and energetics of reptilian and avian embryos. In: Egg incubation: its effects on embryonic development in birds and reptiles (D. C. Deeming and M. W. J. Ferguson, eds.), pp. 285-306. Cambridge University Press, Cambridge.
Vleck, C. M., and D. Vleck. 1987. Metabolism and energetics of avian embryos. Journal of Experimental Zoology, Supplement 1: 111-125.
Wiebe, K. L., W. D. Koenig, and K. Martin. 2006. Evolution of clutch size in cavity-excavating birds: the Nest Site Limitation Hypothesis revisited. American Naturalist 167: 343–353.
Welty, J.C. and L. Baptista. 1998. The life of birds, fourth ed. Saunders College Publishing, New York.
Zelenitsky, D. K., S. P. Modesto, and P. J. Currie. 2002. Bird-like characteristics of troodontid theropod eggshell. Cretaceous Research 23: 297-305.
More lecture notes:
Useful links: