|
Ornithology Avian Reproduction: Anatomy & the Bird Egg |
|
|
Ornithology Avian Reproduction: Anatomy & the Bird Egg |
|
Reproductive Anatomy:
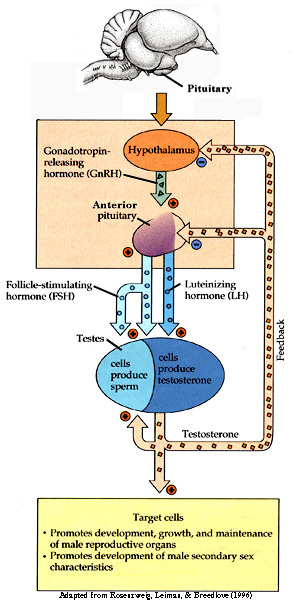
From: Akins and Burns (2001)
A Tale of Two Species:
Carry-over effects and seasonal interactions
Presented By Peter P. Marra
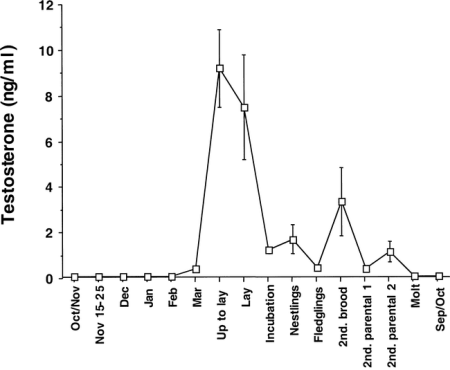
The pattern of testosterone secretion in free-living populations of Song Sparrows.
Plasma levels peak in April and May as breeding got underway and then were maintained at a lower “breeding baseline” during the
rest of the breeding season. As prebasic molt ensued, plasma levels of testosterone were basal and remained so throughout autumn and winter.
From: Wingfield and Hahn (1994).
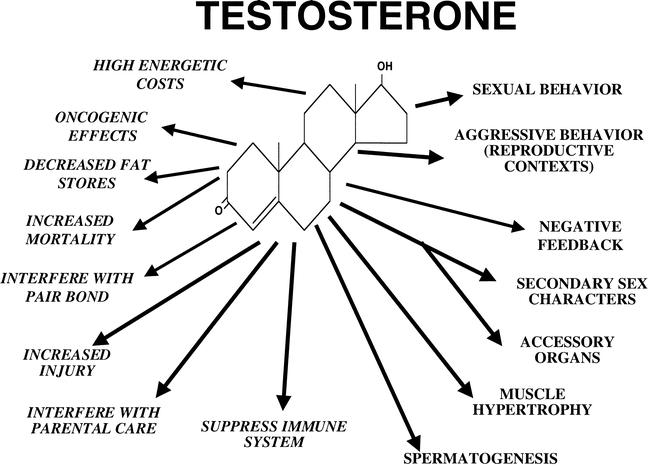
Biological actions of the steroid hormone testosterone. The morphological, physiological and behavioral actions of testosterone that are essential
for male reproductive function are given on the right hand and lower sides of the figure. The “costs” of prolonged high levels of testosterone are given on the
left hand side in italics. The patterns of plasma testosterone levels may be a function of secretion patterns to maintain male reproductive function, and “costs”
of testosterone that require that plasma levels be low. From Wingfield et al. (2000).
| Testosterone increases availability of carotenoids -- Androgens and carotenoids play a fundamental role in the expression of secondary sex traits in animals that communicate information on individual quality. In birds, androgens regulate song, aggression, and a variety of sexual ornaments and displays, whereas carotenoids are responsible for the red, yellow, and orange colors of the integument. Parallel, but independent, research lines suggest that the evolutionary stability of each signaling system stems from tradeoffs with immune function: androgens can be immunosuppressive, and carotenoids diverted to coloration prevent their use as immunostimulants. Despite strong similarities in the patterns of sex, age and seasonal variation, social function, and proximate control, there has been little success at integrating potential links between the two signaling systems. These parallel patterns led us to hypothesize that testosterone increases the bioavailability of circulating carotenoids. To test this hypothesis, Blas et al. (2006) manipulated testosterone levels of Red-legged Partridges (Alectoris rufa) while monitoring carotenoids, color, and immune function. Testosterone treatment increased the concentration of carotenoids in plasma and liver by >20%. Plasma carotenoids were in turn responsible for individual differences in coloration and immune response. These results provide experimental evidence for a link between testosterone levels and immunoenhancing carotenoids that (i) reconciles conflicting evidence for the immunosuppressive nature of androgens, (ii) provides physiological grounds for a connection between two of the main signaling systems in animals, (iii) explains how these signaling systems can be evolutionary stable and honest, and (iv) may explain the high prevalence of sexual dimorphism in carotenoid-based coloration in animals. |  Red-legged Partridge (Photo by G. Bortolotti) |
Sperm production
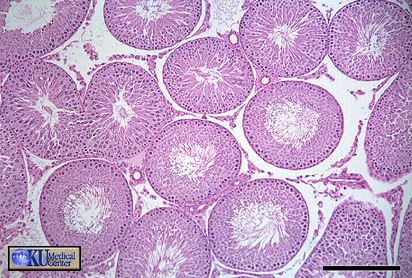
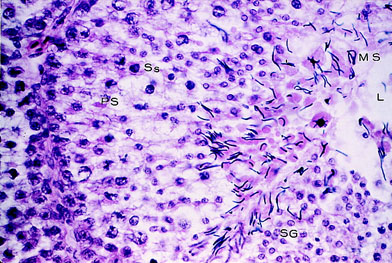
Light photomicrograph of a section of a testis showing
a seminiferous tubule during full
semen production. SG indicates spermatogonia; PS, primary
spermatocyte; Ss, secondary spermatocyte;
MS, mature spermatocyte; and L, lumen (original magnification
×800) (Samour 2002).
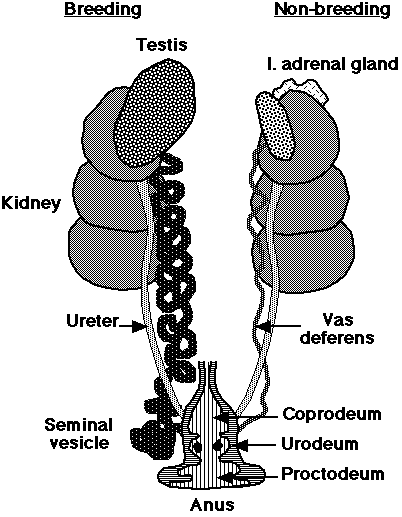
sperm are stored at the terminal end of the vas deferens (seminal glomus), and this creates a swelling called the cloacal protuberance
| Male birds have paired abdominal testes lying cranioventral
to the first kidney lobe. Testes increase dramatically in size during the
breeding season. The vas deferens emerges medially and passes caudally
to the cloaca where it has a common opening with the ureter in the Urodeum.
The terminal vas deferens is swollen as a storage organ: the seminal glomus
(or seminal vesicle as in the drawing to the right).
As in mammals, sperm formation is temperature sensitive, and maturation is assisted by nocturnal drops in temperature, or by the development of scrotal-like external thermoregulatory swellings holding the seminal glomera. In addition, male birds tend to have relatively low extragonadal sperm reserves and sperm are ejaculated soon after production in the testes. |
|
Cloacal protuberance |
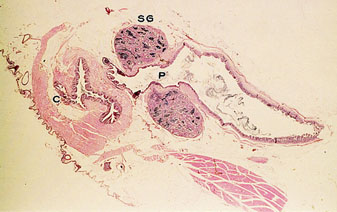
Longitudinal section of the cloaca of a male budgerigar during the culmination phase of the breeding cycle. SG indicates seminal glomus; P, proctodeum; and C, cloaca (original magnification ×12) (Samour 2002). |
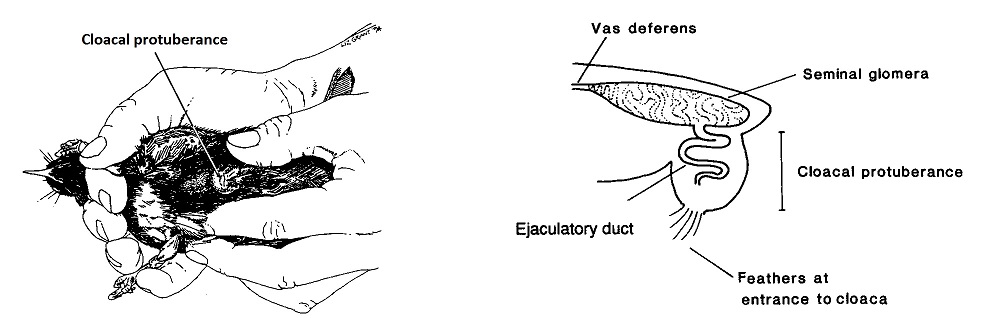
Cloacal protuberance of a male Stitchbird (also call the Hihi) (From: Castro et al. 1996).
Cloacal protuberance is discussed beginning at 3:03
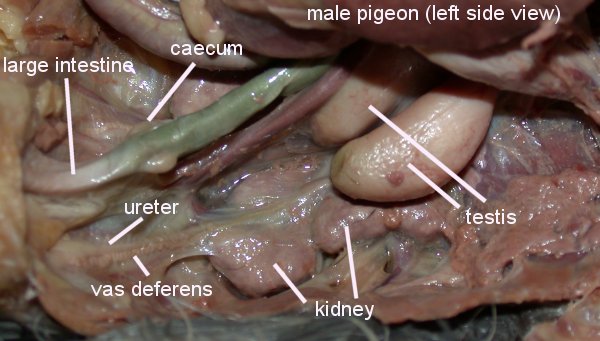
Photo source: http://www.wtamu.edu/~rmatlack/pigeon_dissection/male_reproductive.jpg
Sperm competition and testes size -- Comparative analyses suggest that a variety of ecological and behavioural factors contribute to the
tremendous variability in extrapair mating among birds. In an analysis of 1010 species of birds, Pitcher et al. (2005) examined several ecological
and behavioural factors in relation to testes size; an index of sperm competition and the extent of extrapair mating. In univariate and multivariate
analyses, testes size was significantly larger in species that breed colonially than in species that breed solitarily, suggesting that higher breeding
density is associated with greater sperm competition. After controlling for phylogenetic effects and other ecological variables, testes size was also
larger in taxa that did not participate in feeding their offspring. In analyses of both the raw species data and phylogenetically independent contrasts,
monogamous taxa had smaller testes than taxa with multiple social mates, and testes size tended to increase with clutch size, which suggests that
sperm depletion may play a role in the evolution of testes size. These results suggest that traditional ecological and behavioural variables, such as
social mating system, breeding density and male parental care can account for a significant portion of the variation in sperm competition in birds.
| Testis size increases with colony size in Cliff Swallows -- By using a sample of over 800 male Cliff Swallows (Petrochelidon pyrrhonota) that died during a rare climatic event in their Nebraska study area in 1996, Brown and Brown (2003) investigated how testis size was related to body size, age, parasite load, and a bird's past colony-size history. Testis volume increased with body size. After correcting for body size, testis volume was lowest for birds age 1 and 2 years but did not vary with age for males 3 years old or more. Birds occupying parasite-free (fumigated) colonies had significantly larger testes than did birds at nonfumigated sites. Testis volume increased significantly with the size of the breeding colonies a bird had used in the past. These results show within a species that larger testes are favored in more social environments, probably reflecting a response to increased rates of extrapair copulation (and thus sperm competition) among Cliff Swallows in large colonies. The presence of ectoparasites, by inflating levels of plasma corticosterone, may in turn reduce testis mass. These data provide no support for the hypothesis that large testes, perhaps by producing more testosterone, are immunosuppressive and thus costly for that reason. |
|

Great Tit provisioning nestlings
(Source: http://www.nature.com/)
Temperature and the timing of reproduction -- Many bird species reproduce earlier in years with high spring temperatures, but little is known about the causal effect of temperature. Temperature may have a direct effect on timing of reproduction, but the correlation may also be indirect, for instance via food phenology. As climate change has led to substantial shifts in timing, it is essential to understand this causal relationship to predict future impacts of climate change. Visser et al. (2009) tested the direct effect of temperature on laying dates in Great Tits (Parus major) using climatized aviaries in a 6-year experiment. Temperature patterns from two specific years in which the wild population laid either early (‘warm’ treatment) or late (‘cold’ treatment) were mimicked. Laying dates were affected by temperature directly. Because the relevant temperature period started three weeks prior to the mean laying date, with a range of just 4°C between the warm and the cold treatments, and because the birds were fed ad libitum, it is likely that temperature acted as a cue rather than lifting an energetic constraint on the onset of egg production. Visser et al. (2009) also found a high correlation between the laying dates of individuals reproducing both in aviaries and in the wild, validating investigations of reproduction of wild birds in captivity. These results demonstrate that temperature has a direct effect on timing of breeding, an important step towards assessing the implication of climate change on seasonal timing.
Egg production
Avian Ovary (Source: http://www.ksvea.com/birds.html) |
Source: ulisse.cas.psu.edu/4hembryo/female.html |

Source: ulisse.cas.psu.edu/4hembryo/female.html |
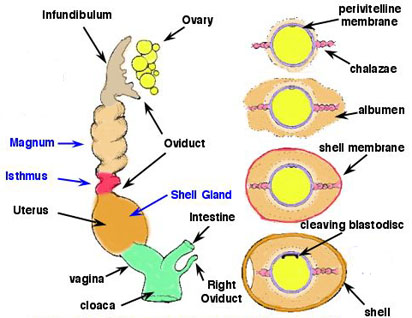
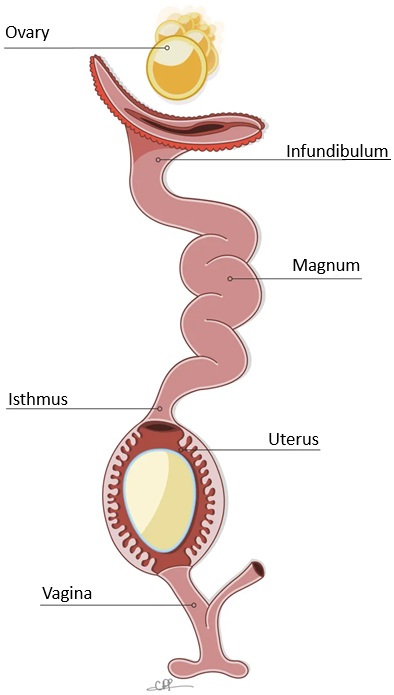
| In most birds, only the left ovary and oviduct
persist. The ovary enlarges greatly during the breeding season. Active
ovaries resemble bunches of tiny grapes -- the developing follicles. The
oviduct opens medially to it in a funnel-shaped ostium. Ovulation results
in the release of an egg from a mature follicle on the surface of the ovary.
The egg, with extensive food reserves in the form of concentric layers
of yolk, is picked
up by the ostium and ciliary currents carry it into the magnum
region. Over about three hours the egg receives a coating of albumen.
The egg then passes into the isthmus, where the shell membranes are deposited. This takes about one hour. The egg them moves to the uterus, or shell gland, where the calcareous shell is added and, in some birds, pigment is added in characteristic patterns. The egg then passes into the vagina and cloaca for laying. |
|
Reproductive system of a female bird, containing an incomplete egg in the uterus.
After ovulation, the vitelline membrane and albumen are deposited during passage
through the infundibulum and magnum, respectively. Next, the egg passes through the
isthmus where the precursors of the eggshell membranes are secreted and assembled.
The resulting meshwork of interlaced fibers is organized into distinct inner and outer
sheets. Finally, the eggshell forms in the uterus (From: Hincke et al. 2012).
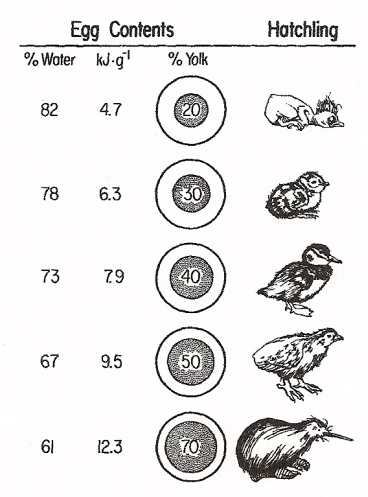
Variation among bird species in the relative amount of yolk in eggs and the amount of energy available to the developing embryo (kJ-g -1, or kilojoules per gram). From top to bottom, the hatchlings are an altricial Brown Creeper, a semiprecocial Least Tern, a precocial Ruddy Duck, a superprecocial Mallee Fowl (Leipoa ocellata), and a Brown Kiwi (Apteryx australis). Kiwis are ‘outliers.’ Female kiwis produce extremely large eggs for their size (with substantial amounts of yolk), but young typically remain in the nest for several days and so are best classified as semiprecocial (From: Sotherland and Rahn 1987).
Kiwi lays an egg

texture of the shell pieces probably resembles the original texture of the egg. Credit: Yen-nien Cheng |
Eggs discovered inside dinosaur -- The discovery
of eggs inside a dinosaur has provided new clues about dinosaur reproductive
biology and more support for the hypothesis that birds evolved from dinosaurs.
The pair of eggs are the first found inside a dinosaur. Sato et al. (2005)
found that the dinosaur produced eggs in some ways like a crocodile and
in other ways like a bird. Crocodiles have two ovaries enabling them to
lay a clutch of eggs. Birds have a single ovary and lay only one egg at
a time. The dinosaur's egg-producing capability lay somewhere in between,
suggesting a possible link with modern birds. It had two ovaries, but produce
only one egg at a time from each ovary.
Sato et al. (2005) studied a dinosaur from a group of dinosaurs called oviraptorosaurians. This type of dinosaur — probably 3 - 4 meters long — is a subgroup of the theropods. The dinosaur was excavated in China. The similar size of the eggs suggests the creature's two oviducts each produced a single egg at the same time. |
Female
birds can bias the sex of their chicks.-- Whether a bird is more
likely to lay a male or female egg depends on which sex will have the greatest
chance of doing well. Rutstein et al. (2004) adjusted the food intake of
female Zebra Finches [see photo of female (left) and male (right) Zebra
Finches below right] & found that well-fed females were more likely
to produce daughters, while less well nourished birds were more likely
to have sons. This is exactly as predicted by the fact that female offspring
need to be better nourished than males if they are to survive and grow
well. 
The authors noted that: “In most animals sex ratio is close to 50:50 and extremely resistant to change. In mammals, including humans, the sex of the baby is determined by whether the sex chromosome in the sperm is male or female. But in birds, it is the female’s egg rather than the male’s sperm that determines what sex the chick will be. Thus the female has the potential to determine the sex of her young by whether she ovulates male or female eggs. In some way, female Zebra Finches seem to be able to exert control over whether to produce a male or female egg depending on which of the two is most likely to be successful. Our research tells us that they do it, and we understand why. The big question is: how do they do it?” In many animals, females need to be well-nourished and in good condition if they are to breed, as eggs are costly to produce. Bigger eggs tend to lead to bigger young that are more likely to survive. Such ‘sex ratio adjustment’ is well documented in certain insects, such as bees and wasps, but is less well understood in birds and mammals. Birds are an excellent model to use in the study of sex ratio adjustment because, using molecular techniques, scientists can establish the sex of each egg soon after laying. Further, all the resources given to the developing embryo are present in the egg at laying. Thus the size and the content of the egg are measures of the amount of resources that the female has allocated to that egg, which affects its subsequent survival chances. The authors explained: “We manipulated the diet quality of Zebra Finches to look at the effects of body condition on female investment. We found that females were able to exert a strong degree of control over the production of male and female eggs. When females were fed on a low quality diet, they laid eggs that were considerably lighter than those laid when they were fed on a high quality diet, and they also laid far more male eggs on a low quality diet. This is the converse situation to that described 30 years ago for mammals, but it makes sense for Zebra Finches. Previous research has shown that under poor nutritional conditions, female Zebra Finches grow more slowly and survive less well compared to males. Therefore, females are producing more of the sex with the highest survival chances under those conditions.” |
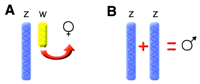
Two potential mechanisms for sex determination among birds. (A) the presence of the W chromosome triggers femaleness or (B) the presence of two Z chromosomes confers maleness. |
Avian sex determination (Ellegren 2001) -- The
molecular determinants behind sexual development in birds remain a mystery.
The process is known to be different from that in mammals, with no homolog
to the gene that confers maleness in mammals found in birds. The failure
to identify such a gene in birds is probably a reflection of the fact that,
despite the occurrence of two sexes being nearly universal throughout the
animal kingdom, the genes involved seem virtually unrelated among metazoan
phyla. These differences raise obstacles for comparative or candidate gene
approaches in studies of sexual development.
In birds, females are the heterogametic sex, with one copy each of the Z and W sex chromosomes. Males are homogametic (ZZ). However, it is not clear whether it is the presence of the female-specific W chromosome that triggers female development, or the dose of Z chromosome that confers maleness. An intriguing additional possibility is that both Z and W matter! In marsupials, for example, Y acts as a dominant testis determining chromosome, while the X chromosome determines the choice between pouch and scrotum. Maybe a system where the two sex chromosomes mediate different aspects of sex differentiation is also used in birds. |
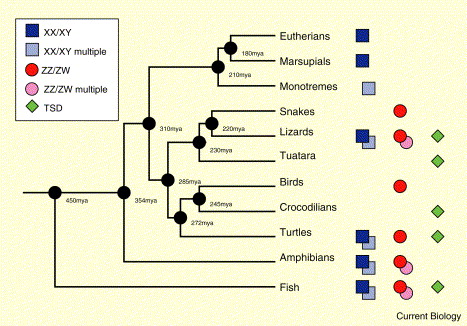
Vertebrate sex determination systems. Phylogeny of major vertebrate clades showing the sex determining systems
found in members of the respective clade. ‘Multiple’ indicates involvement of more than one pair of chromosomes in sex determination.
TSD: temperature-dependent sex determination (From: Ezaz 2006).
Incubation temperature and avian sex ratios -- Although common in reptiles, incubation temperature has not been considered to be a factor in determining sex ratios  in birds. However, Goth and Booth (2005) found that incubation temperature does affect sex ratios in megapodes, which are exceptional among birds because they use environmental heat sources for incubation. In the Australian Brushturkey (Alectura lathami), a mound-building megapode, more males hatch at low incubation temperatures and more females hatch at high temperatures, whereas the proportion is 1:1 at the average temperature found in natural mounds. Chicks from lower temperatures weigh less, which probably affects offspring survival, but are not smaller. Megapodes possess heteromorphic sex chromosomes like other birds, which eliminates temperature-dependent sex determination, as described for reptiles, as the mechanism behind the skewed sex ratios at high and low temperatures. Instead, Goth and Booth (2005) suggest a sex -biased temperature-sensitive embryo mortality because mortality was greater at the lower and higher temperatures, and minimal at the middle temperature where the sex ratio was 1:1.
in birds. However, Goth and Booth (2005) found that incubation temperature does affect sex ratios in megapodes, which are exceptional among birds because they use environmental heat sources for incubation. In the Australian Brushturkey (Alectura lathami), a mound-building megapode, more males hatch at low incubation temperatures and more females hatch at high temperatures, whereas the proportion is 1:1 at the average temperature found in natural mounds. Chicks from lower temperatures weigh less, which probably affects offspring survival, but are not smaller. Megapodes possess heteromorphic sex chromosomes like other birds, which eliminates temperature-dependent sex determination, as described for reptiles, as the mechanism behind the skewed sex ratios at high and low temperatures. Instead, Goth and Booth (2005) suggest a sex -biased temperature-sensitive embryo mortality because mortality was greater at the lower and higher temperatures, and minimal at the middle temperature where the sex ratio was 1:1.
Copulation & fertilization:

Common Kingfishers mating
| Phony phallus puts sperm ahead in bird first-- "These birds would be at it for 10-20 minutes," said co-author Tim Birkhead of the Red-billed Buffalo Weaver. Males use their organ to rub females and improve their sperm's chance of success. Few male birds have a phallus; most achieve fertilization via a cloacal kiss. So 19th-century reports of a mock member in the Buffalo Weaver sent Winterbotton et al. (2001) to Namibia. Catching the birds in the act was tough, recounts Birkhead: "In 3 years we saw eight matings." Pairs occasionally emerged from nests and flew to a nearby tree. "I'd run after them, sweating profusely with my binoculars steaming up," he says. The pair would start bouncing up and down - over numerous consecutive bouts. Compared to the 1-2 second tryst of most birds, their staying power is unique. Yet, entry of the elusive organ was hard to make out. Even in captivity "they performed beautifully," but the view was blocked, says Birkhead. So they glued a piece of cardboard to an unlucky bird's member. This did not prevent mating, suggesting that the Buffalo Weaver organ is actually a weapon in sperm wars. By choosing a male who rubs longest or best, females may be selecting top-quality sperm. Paternity testing revealed that female Buffalo Weavers sire birds from multiple males, providing evidence of sperm competition. Time spent courting must be shown to predict sperm transfer or success to really back up the idea. The 1.5-cm appendage lacks blood vessels and has a twisted furrow down its length. Males in communal nests have longer ones than those that live alone, showing that size is a factor in social success. But for males at least, the phallus is for more than foreplay. -- Helen Pearson, Nature Science Update |
|
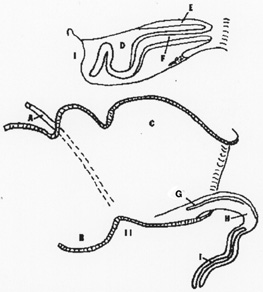
Diagram of the left lateral view of a retracted and erect
phallus of a male Emu or Rhea. The top drawing
represents the phallus within
the pouch. A. vas deferens, B. urideum, C. proctodeum, D. pocket to contain
phallus,
E. erectile wall of phallus, F inverted hollow tube of
phallus, G. phallic sulcus, H. erectile tissue, and I. erect phallus
with blind hollow tube.
Genital Evolution in Birds: Losing the Penis & Winning the Battle
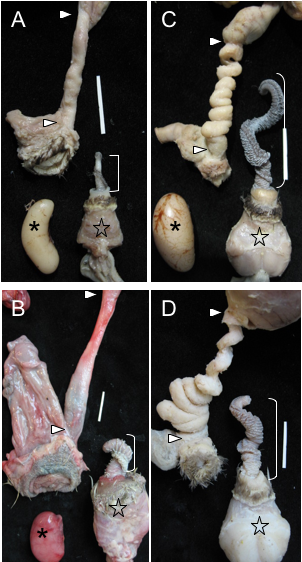
Examples of genital covariation in waterfowl.
(A) Harlequin Duck (Histrionicus histrionicus) and (B) African Goose (Anser cygnoides), two species with a short phallus and no forced copulations, in which females have simple vaginas. (C) Long-tailed Duck (Clangula hyemalis), and (D) Mallard (Anas platyrhynchos), two species with a long phallus and high levels of forced copulations, in which females have very elaborate vaginas (size bars = 2 cm). ] = Phallus, * = Testis, star = Muscular base of the male phallus, > = upper and lower limits of the vagina (From: Brennan et al. 2007).
Eversion of a male Muscovy duck penis
Explosive eversion and functional morphology of the duck penis -- Coevolution of male and female genitalia in waterfowl has been hypothesized to occur through sexual conflict. This hypothesis raises questions about the functional morphology of the waterfowl penis and the mechanics of copulation in waterfowl. Brennan et al. (2010) used high-speed video of phallus eversion and histology to describe for the first time the functional morphology of the avian penis. Eversion of the 20 cm muscovy duck penis is explosive, taking an average of 0.36 sec, and achieving a maximum velocity of 1.6 m sec−1. The collagen matrix of the penis is very thin and not arranged in an axial-orthogonal array, resulting in a penis that is flexible when erect. To test the hypothesis that female genital novelties make intromission difficult during forced copulations, Brennan et al. (2010) investigated penile eversion into glass tubes that presented different mechanical challenges to eversion. Eversion occurred successfully in a straight tube and a counterclockwise spiral tube that matched the chirality of the waterfowl penis, but eversion was significantly less successful into glass tubes with a clockwise spiral or a 135° bend, which mimicked female vaginal geometry. These results support the hypothesis that duck vaginal complexity functions to exclude the penis during forced copulations, and coevolved with the waterfowl penis via antagonistic sexual conflict.
Near the junction of the vagina and shell gland of female birds are
deep glands lined with simple columnar epithelium. These are the sperm
storage tubules, so called because they can store sperm for long periods
of time (10 days to 2 weeks). After an egg is laid, some of these sperm
may move out of the tubules into the lumen of the tract, then migrate farther
up to fertilize another egg.
Avian sperm |

Avian sperm storage tubules |
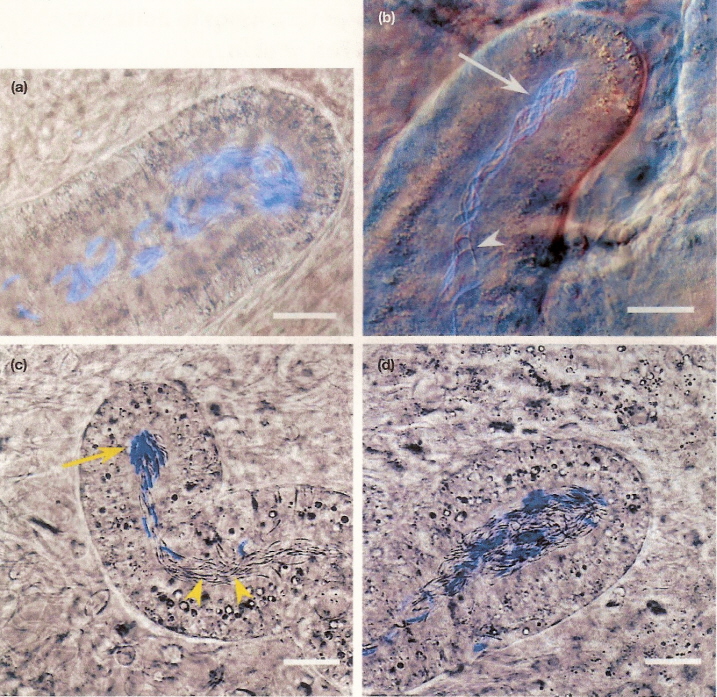
Photomicrographs of sperm storage tubules contained stained and unstained spermatozoa from domestic chicken (Gallus domesticus)
hens (a, b)
and turkey (Meleagris gallopavo) hens (c, d). Arrows indicate stained spermatozoa; arrowheads designate unstained spermatozoa.
Scale bars = 25 micrometers.
From: King et al. (2002).
King et al. (2002) found that spermatozoa from two different inseminations (one with stained sperm, one with unstained sperm) generally
segregated into different storage tubules in both chicken and turkey hens. Storage tubules contained mixed populations of spermatozoa were
found in only 4% of chicken and 12% of turkey storage tubules examined. They concluded that the mechanism of last-male precedence does
not appear to be due to the stratification of spermatozoa within the tubules.
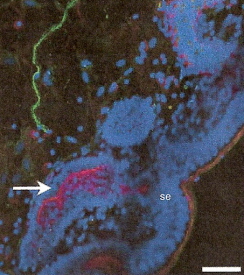 |
Innervation of sperm storage tubules (Freedman et al. 2001) -- Immunohistochemical staining of a turkey uterovaginal junction and sperm storage tubules. This micrograph shows a fluorescing neuron (green) near some sperm storage tubules (SST). The blue areas (se) are the surface epithelium lining the lumen of the uterovaginal junction and the epithelium of the sperm storage tubules. The arrow points to a magenta-stained area of one SST that indicates the presence of actin (a protein found in smooth muscle. The total image is 19 micrometers across. This association between neurons and SSTs provides evidence that SSTs are innervated and suggests that the storage and release of sperm from SSTs can, perhaps, be controlled. |
Post-insemination events (Birkhead and Brillard 2007) -- Most birds do not have a phallus and, in these species, insemination occurs via the so-called ‘cloacal kiss.’ Depending on taxa, sperm are ejaculated into the cloaca or vagina and rely on their motility to reach the numerous sperm-storage tubules (SSTs) located at the junction of the vagina and the uterus. As a consequence of selection during their migration through the vagina, only 1–2% of inseminated sperm enter the SSTs, the rest are probably ejected the next time that the female defecates. The SSTs contain only morphologically normal sperm, suggesting either that only normal sperm successfully traverse the vagina or that only normal sperm are ‘accepted’ by the SSTs. An unknown but probably small proportion of sperm move directly to the infundibulum (the site of fertilization) without entering the SSTs, although these are likely to fertilize only a single ovum.
That sperm in the SSTs are invariably positioned with their heads directed towards the distal end of the tubule suggests that egress from the SSTs is passive. Sperm are lost from the SSTs more or less continuously at a constant per capita rate. They enter the uterus and are carried passively to the infundibulum. Sperm accumulate or move relatively slowly through the infundibulum so that there is usually a population available to fertilize each ovum as it is ovulated. On ovulation, the ovum is captured by the prehensile, funnel-shaped infundibulum and the sperm swarm over the surface of the ovum; their target is the germinal disc, which contains the female pronucleus. At this stage, the ovum is bounded by the inner perivitelline layers (IPVL). The clustering of sperm and holes made by sperm in the IPVL around the germinal disc suggest that sperm might use chemical signals to locate the germinal disc.
In contrast to most other taxa, where only a single sperm enters the ovum, polyspermy is typical in birds. Several sperm enter the germinal disc region, hydrolyzing the IPVL via the acrosome reaction of the sperm, whereby the release of enzymes from the sperm acrosome enables the sperm nucleus to enter the ovum. However, only a single spermatozoon fuses with the female pronucleus and the remaining sperm are shifted to the periphery of the germinal disc and play no further part in development. Fertilization includes the penetration of ovum by sperm as well as the fusion of the male and female pronuclei (syngamy). Because embryo development begins almost immediately, many cell divisions have occurred by the time the ovum has become incorporated into the egg and the egg is laid (in most species) 24 hr later.
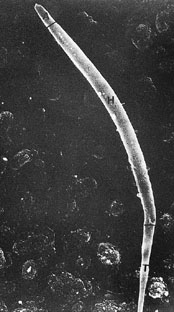
Scanning electron photomicrograph of a budgerigar spermatozoon. A indicates acrosome; H, head; and T, tail (original magnification ×20000) (Samour 2002). |
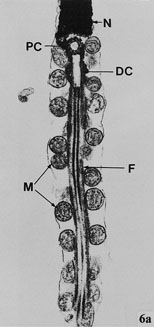
Transmission electron photomicrograph of the longitudinal section of part of the nucleus and midpiece of a Budgerigar spermatozoon. N indicates nucleus; PC, proximal centriole; DC, distal centriole; F, axial filament complex; and M, mitochondria (original magnification ×30000) (Samour 2002). |
Fertilization of the egg usually occurs in the infundibulum.
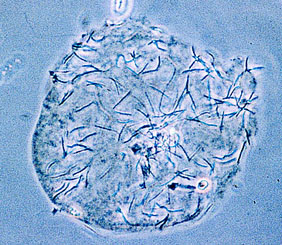
Light photomicrograph of a zona-free hamster ovum with
numerous budgerigar spermatozoa bound to its surface
(original magnification ×215) (Samour 2002).
| Repelling clingy exes helps snipe save sperm -- Writer Gore Vidal once said that he never passed up an opportunity to have sex or appear on television. Some male birds would disagree on at least one count. Having mated with a female, a male Great Snipe (Gallinago media) will reject her further advances and even chase her away. Male Great Snipe form leks to eye up the talent before choosing a mate. A few males get the most sex. Popular birds can get more than half of the matings, perhaps 10 a day. Hence their pickiness, suggest Saether et al. (2001). As male Great Snipe take no part in caring for their offspring, it was thought they had nothing to lose by mating as much as possible. But top males, overburdened with potential partners, must share sperm with care and spread their favors around. Sperm budgeting is the only possible explanation for male snipes' ungrateful behavior. Like a nightclub, Great Snipe leks see their share of aggravation. "All four kinds of mating conflicts happen" - male choice, female choice, and male and female competition - explains Saether. Males are more likely to repel clingy exes if there are a lot of other females around. Females fight with one another, and males from neighboring territories chase their rivals' females away. Hostility towards old flames might be a bid to maintain order. "If a male gets rid of an unwanted female it's one less problem to worry about," says Saether. Female snipe probably seek to mate again so that they can get enough sperm to fertilize their eggs. Rejected females tend to lower their sights and settle for less popular males. -- John Whitfield, Nature Science Update | 
www.nature.com/nsu/011018/011018-8.html |
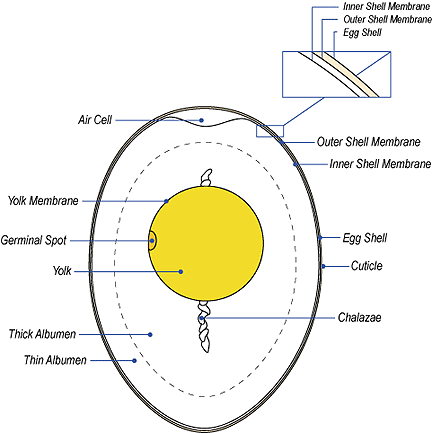
The Avian Egg
Birds' eggs, like the birds themselves, vary enormously in size. The
largest egg from a living bird belongs to the ostrich. It is over 2000 times larger than the smallest egg produced by a hummingbird (see
photo to the right).
Ostrich eggs are about 180 mm long and 140 mm wide and weigh 1.2 kg. Hummingbird
eggs are 13 mm long and 8 mm wide and they weigh only half of a gram. The
extinct Elephant Bird from Madagascar produced an egg 7 times larger than
that of the Ostrich!
2000 times larger than the smallest egg produced by a hummingbird (see
photo to the right).
Ostrich eggs are about 180 mm long and 140 mm wide and weigh 1.2 kg. Hummingbird
eggs are 13 mm long and 8 mm wide and they weigh only half of a gram. The
extinct Elephant Bird from Madagascar produced an egg 7 times larger than
that of the Ostrich!
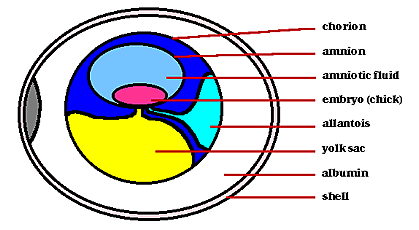
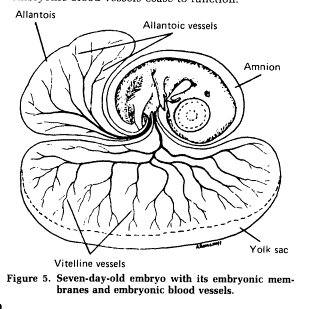
Within the egg, three extraembryonic membranes support the life & growth of the embryo:
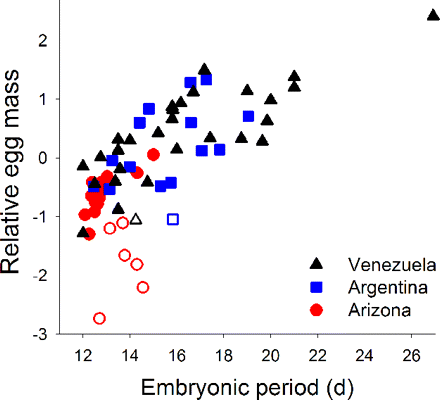
Relative egg mass (corrected for adult mass) is greater in species with longer embryonic periods (days) among 64 passerine species in tropical Venezuela, subtropical Argentina, and north temperate Arizona. Open symbols reflect cavity-nesting species and show an interacting effect where their larger clutches are associated with relatively smaller eggs.
Egg size variation among tropical and temperate songbirds -- Species with “slow” life history strategies (long life, low fecundity) are thought to produce high-quality offspring by investing in larger, but fewer, young. Larger eggs are indeed associated with fewer eggs across taxa and can yield higher-quality offspring. Tropical passerines appear to follow theory because they commonly exhibit slow life history strategies and produce larger, but fewer, eggs compared with northern species. Martin (2008) found that relative egg mass (corrected for adult mass) varies extensively in the tropics and subtropics for the same clutch size, and proposed a hypothesis to explain egg size variation both within the tropics and between latitudes: Relative egg mass increases in species with cooler egg temperatures and longer embryonic periods to offset associated increases in energetic requirements of embryos. Egg temperatures of birds are determined by parental incubation behavior and are often cooler among tropical passerines because of reduced parental attentiveness of eggs. Cooler egg temperatures and longer embryonic periods explained the enigmatic variation in egg mass within and among regions, based on field studies in tropical Venezuela (36 species), subtropical Argentina (16 species), and north temperate Arizona (20 species). Alternative explanations were not supported. Thus, large egg sizes may reflect compensation for increased energetic requirements of cool egg temperatures and long embryonic periods that result from reduced parental attentiveness in tropical birds.
| Egg composition and hatchling phenotype -- Parental investment in eggs and, consequently, in offspring can profoundly influence the phenotype, survival and evolutionary fitness of an organism. Avian eggs are excellent model systems to examine maternal allocation of energy translated through egg size variation. Dzialowski1and Sotherland (2004) used the natural range in Emu (Dromaius novaehollandiae) egg size, from 400 g to >700 g, to examine the influence of maternal investment in eggs on the morphology and physiology of hatchlings. Female Emus provisioned larger eggs with a greater absolute amount of energy, nutrients and water in the yolk and albumen. Variation in maternal investment was reflected in differences in hatchling size, which increased isometrically with egg size. Egg size also influenced the physiology of developing Emu embryos, such that late-term embryonic metabolic rate was positively correlated with egg size and embryos developing in larger eggs consumed more yolk during development. Large eggs produced hatchlings that were both heavier (yolk-free wet and dry mass) and structurally larger (tibiotarsus and culmen lengths) than hatchlings emerging from smaller eggs. As with many other precocial birds, larger hatchlings also contained more water, which was reflected in a greater blood volume. Emu maternal investment in offspring, measured by egg size and composition, is significantly correlated with the morphology and physiology of hatchlings and, in turn, may influence the success of these organisms during the first days of the juvenile stage. |

abc.net.au/schoolstv/animals/EMUS.htm |
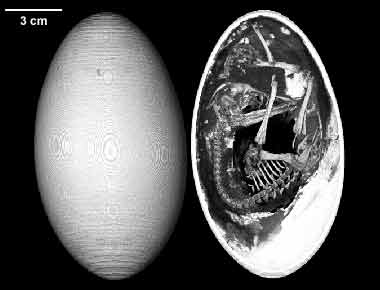
Emu egg & embryo
http://www.digimorph.org/specimens/Dromaius_novaehollandiae/egg/
| Yolk contains maternal antibodies -- Antibodies are deposited in eggs during yolk formation through the deposition of immunoglobulins, primarily IgY (also called IgG), in the yolk. In Chickens (Gallus domesticus), maternal IgY is catabolized by offspring over the first 14 days post-hatching and, by about 5 days post-hatching, offspring begin to synthesize their own IgY. As a result, after approximately two weeks the circulating IgY in young is principally of endogenous origin. Adult levels are attained between six weeks and six months of age. However, maternal antibodies may continue to affect offspring phenotype even after they are catabolized by influencing growth and developmental rates. In the absence of maternal IgY in chickens (due to surgical bursectomy of the mother during her own embryogenesis), the number of cells in the spleen that help lymphocytes (helper T cells) attack antigens (foreign proteins on pathogens) is depressed. Also, the immune responsiveness of offspring is depressed, which could lower the survival of offspring particularly in harsh disease environments (Grindstaff et al. 2003). |
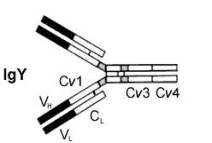
Antibodies 'attack' pathogens or toxins they produce by binding to antigens (e.g., proteins in the membranes of bacteria) via their 'binding sites' (the black areas above). This binding can neutralize toxins and attract white blood cells that eliminate pathogens (by phagocytosis). |
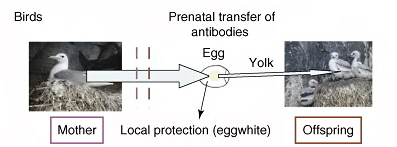
Maternal secretion of antibodies and absorption by the young occur only prenatally in birds (with the exception of pigeon crop milk)
(From: Boulinier and Staszewski 2008).

The familiar color of a chicken’s egg yolk (a) is in stark contrast to the richly pigmented egg yolk of a lesser Black-backed Gull, Larus fuscus (b). Such high maternal investment of carotenoids into egg yolk is typical among wild bird species, suggesting that these biologically active pigments serve important functions in the developing bird (From: Blount et al. 2000). |
Why egg yolk is yellow (or red) (Blount et al. 2000) -- Egg yolk in birds is colored yellowish-red by carotenoids. Until recently, there has been no adaptive explanation of why many egg-laying animals provision their eggs so richly with carotenoids. It now appears that, in developing birds, carotenoids protect vulnerable tissues against damage caused by free radicals. Athough embryonic tissues depend on oxidizable, unsaturated fatty acids in yolk, their abundance makes the tissues susceptible to peroxidation caused by reactive oxidative metabolites and by free radicals, which are produced as normal by-products of metabolism. Protection against lipid peroxidation in young birds is afforded by the actions of yolk-derived carotenoids and other antioxidants, like vitamin E. Antioxidants also protect passively-acquired antibodies (IgY; see above) against break-down. Thus, maternal investment in egg composition, including carotenoids, might have a greater influence on offspring viability than has been realized. The use of carotenoid pigments in the sexual displays of female birds might indicate their ability to produce high quality eggs and chicks. |

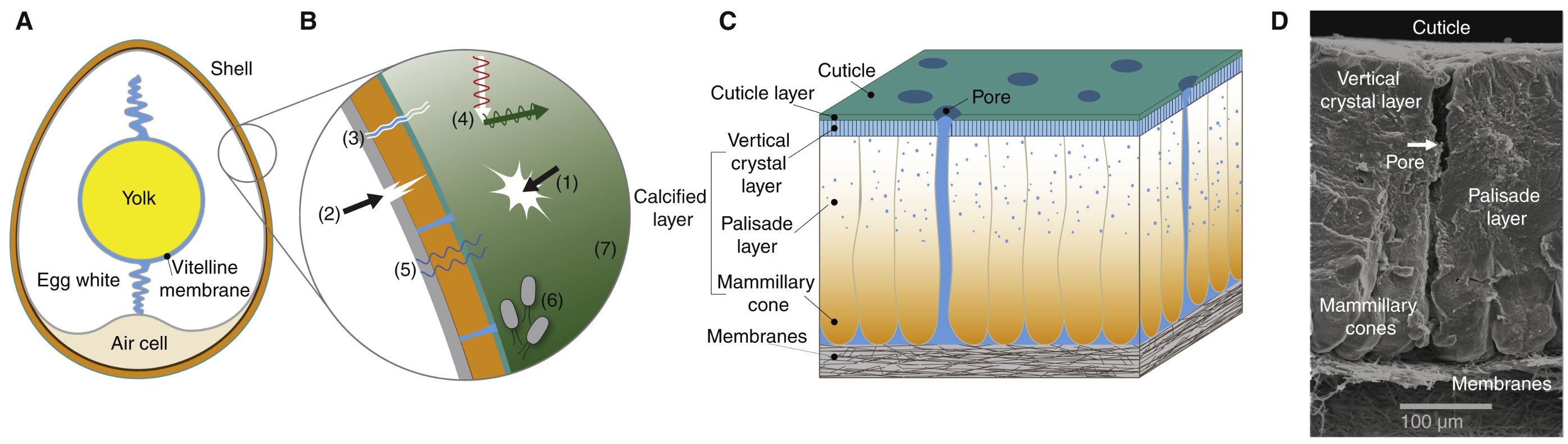
Structure and multifunctionality of the avian eggshell. (A) Longitudinal cross-section of an egg. (B) Diagram illustrating the many functions of the shell,
including: (1) resisting mechanical damage; (2) facilitating crack propagation from the inside during hatching; (3) permitting gas (oxygen and carbon dioxide)
exchange; (4) regulating thermal properties; (5) mediating water loss; (6) minimizing microbial invasion; and (7) displaying color for signaling and camouflage.
(C) Schematic diagram of chicken eggshell microstructure and (D) corresponding cross-sectional scanning electron micrograph. At the innermost part of the
eggshell, there are two shell membranes (C,D). Next comes the mammillary cone layer (where calcium carbonate crystals start to grow in conical protrusions),
the palisade layer (the thickest part of the shell), the outer vertical crystal layer and finally the cuticle (C,D). Embedded throughout the shell is a matrix of
organic proteins. Tiny pores traverse the shell, allowing for transport of gases (oxygen, carbon dioxide and water vapor) (From: Stoddard 2022).
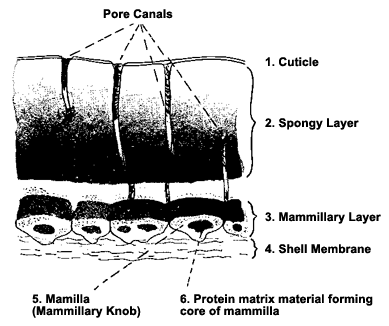


Thousands of tiny pores like the one pictured above,
cover the shell, providing a passage for gas exchange.
(Source: http://www.rit.edu/~tld0898/SEM.html)
| Weaker Birds Use Steroids to Boost Offspring -- Verboven et al. (2003) reported that female gulls in poor condition were more likely to give their chicks a hormone boost to improve their chances of survival. Verboven and her colleagues experimentally enhanced maternal condition by supplementary feeding Lesser Black-backed Gulls (Larus fuscus) during egg formation and compared the concentrations of steroids (including testosterone) in their eggs with those in eggs laid by control females. Egg androgens could affect offspring performance directly through chick development and/or indirectly through changes in the competitive ability of a chick relative to its siblings. Contrary to expectation, females with experimentally enhanced body condition laid eggs with lower levels of androgens. This suggests that less healthy females pass on more steroids than healthy ones in a bid to enhance the performance of their young. Verboven noted that “We originally thought that gulls in good condition would put more steroids in their eggs. But we discovered that healthy birds don’t tend to give their eggs the extra boost.” She compared the situation to struggling athletes who take performance-enhancing drugs. She added: “A poor sports person maybe wants to use steroids to conceal poor performance but if you are good you don’t need to use them.” |

Lesser Black-backed Gulls © Arthur Grosset |
| Avian mothers create different phenotypes by hormone deposition in their eggs -- In birds, mothers deposit substantial amounts of androgens in their eggs, and experimental evidence indicates that these maternal androgens influence the chick's early development. Despite the well-known organizing role of sex steroids on brain and behavior, studies on avian maternal egg hormones almost exclusively focus on the chick phase. Eising et al. (2006) found that, in Black-headed Gulls, maternal androgens in the egg enhance the development of the nuptial plumage and the frequency of aggressive and sexual displays (see Figure above) almost 1 year after hatching.
The long-lasting effects may be mediated by an upregulation of androgen receptors later in life. Alternatively, the
early hormone exposure may have influenced the hypothalamus-pituitary-gonad axis, resulting in higher
androgen production later in life.
The long-lasting effects of egg androgens are almost certainly
beneficial for Darwinian fitness. Successful territory establishment
and defense by means of aggressive interactions are essential for
reproductive success in this colonial breeder. In addition, the
displays are important for mate selection.
Clearly, in birds, maternal hormone deposition in eggs may profoundly influence individual differentiation of fitness related
traits. Since these hormones suppress early immune function of the chick and reduce long-term survival, mothers may be faced with a trade-off between producing offspring with lower survival prospects but higher reproductive success per year, or with higher chances of survival and lower annual reproductive output. By producing eggs that differ in levels of maternal hormones, mothers seem to produce a variety of phenotypes, perhaps an adaptive strategy in unpredictable environmental conditions. Since natural selection acts upon such phenotypic variation, shaping a population's demography, the role of maternal androgens in this selective process may be much greater than anticipated until now. |
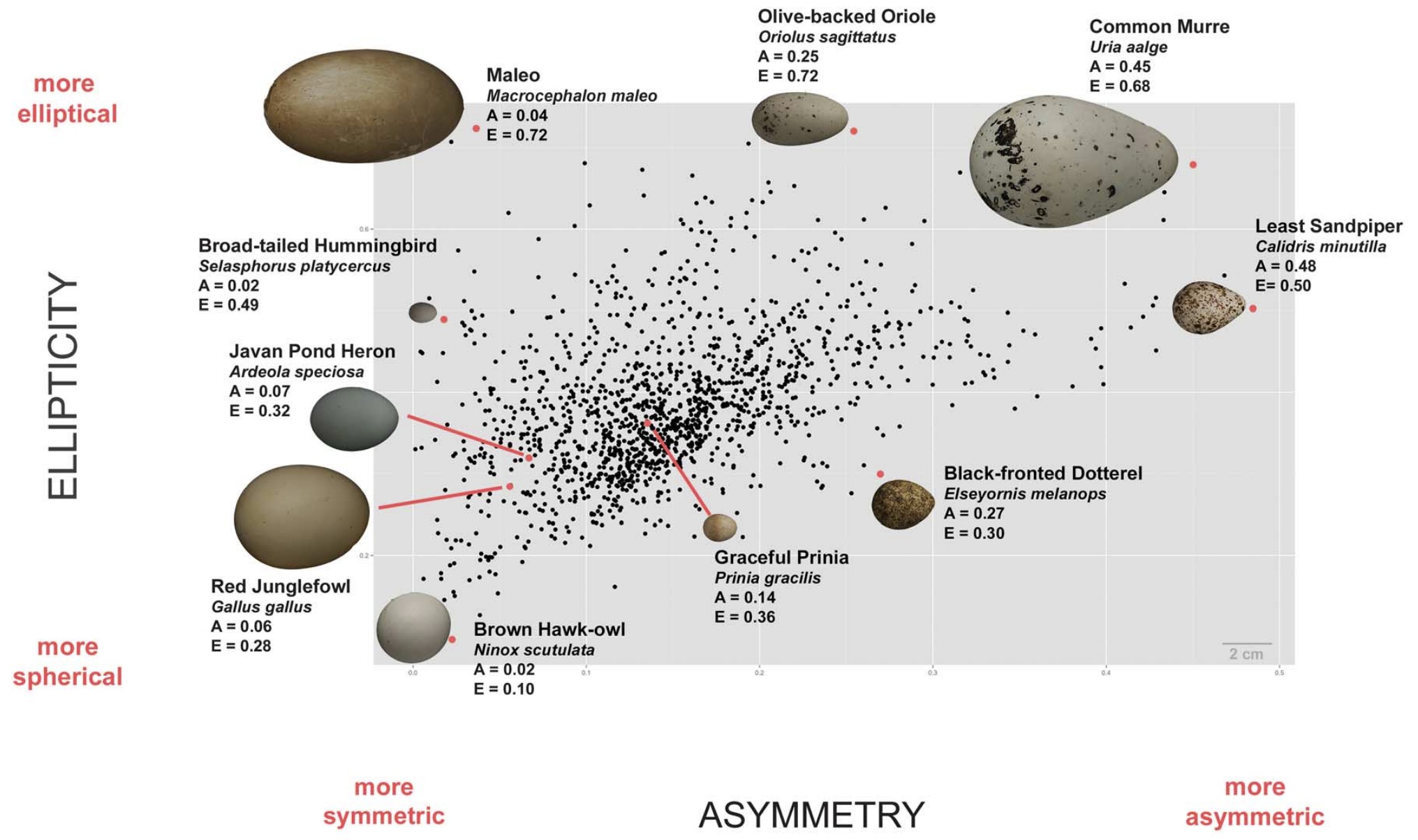
Average egg shapes of 1400 species of birds (black dots) showing variation in asymmetry (A) and ellipticity (E). Images of several
representative eggs are also shown. For A, higher values = more asymmetric. For E, higher values = more elliptical (ranging
from 0 [circle] to values approaching 1 [more elongated]) (From: Stoddard et al. 2017).
Why do bird eggs have different shapes?
The Ecology of Egg Colors
Egg colors and markings have strong adaptive values. Originally, birds' eggs were probably all white, as reptile eggs are. Eggs that are laid on the ground or in open nests in trees, rather than in cavities, often exhibit cryptic coloration. The eggs blend in with their surroundings and are much less visible to potential predators, e.g., Killdeer eggs.

Killdeer nest and eggs
Sometimes eggs that are laid in open nests are white at first. They then become stained by the mud and rotting vegetation in the nest. Grebes lay white eggs that become stained and cryptically colored over time.

Red-necked Grebe nest
Source: http://www.wetlands.org/programs/RussiaCD/eng/3/32/321/red-05.htm
In some species, such as the Common Murre, where different females lay eggs with very different markings, the uniqueness may have a purpose. Distinctive patterns, as in the eggs shown below, help females identify their own egg in a colony where thousands of eggs may dot a cliff face.

Source: http://www.absc.usgs.gov/research/seabird_foragefish/seabirds/flash_cards/common_murre.html
Eggs of kingfishers and other cavity nesting birds, such as woodpeckers and some owls, are often white. The brightness of the eggs may help the parents to more easily locate them in the cavity. Shown here is the egg of a Barn Owl.

Source: http://www.amonline.net.au/birds/gallery/eggs.htm

http://www.skullsunlimited.com/bird-eggs.htm
Evolution of egg color and patterning in birds -- Avian eggs differ so much in their color and patterning from species to species that any attempt to account for this diversity might initially seem doomed to failure. Kilner (2006) reviewed the literature that, when combined with the results of some comparative analyses, suggests that just a few selective agents can explain much of the variation in egg appearance. Ancestrally, bird eggs were probably white and immaculate. Ancient diversification in nest location, and hence in the clutch's vulnerability to attack by predators, can explain basic differences between bird families in egg appearance. The ancestral white egg has been retained by species whose nests are safe from attack by predators, while those that have moved to a more vulnerable nest site are now more likely to lay brown eggs, covered in speckles, just as Wallace hypothesized more than a century ago. Even blue eggs might be cryptic in a subset of nests built in vegetation. It is possible that some species have subsequently turned these ancient adaptations to new functions, for example to signal female quality, to protect eggs from damaging solar radiation, or to add structural strength to shells when calcium is in short supply. The threat of predation, together with the use of varying nest sites, appears to have increased the diversity of egg coloring seen among species within families, and among clutches within species. Brood parasites and their hosts have probably secondarily influenced the diversity of egg appearance. Each drives the evolution of the other's egg color and patterning, as hosts attempt to avoid exploitation by rejecting odd-looking eggs from their nests, and parasites attempt to outwit their hosts by laying eggs that will escape detection. This co-evolutionary arms race has increased variation in egg appearance both within and between species, in parasites and in hosts, sometimes resulting in the evolution of egg color polymorphisms. It has also reduced variation in egg appearance within host clutches, although the benefit thus gained by hosts is not clear.
On the Nature, Ecology, and Evolution of Egg Color

Many small songbirds have eggs with just a ‘ring’ of small spots around the broad end that does little to make the eggs cryptic. Evidence now suggests that such spots are located where the eggshell is a bit thinner (likely due to a calcium deficiency in the diet of female birds), with the pigment serving to strengthen the shell (Gosler et al. 2005). The spots consist of protoporphyrin pigment that birds synthesize during production of the heme component of hemoglobin (Burley and Vadhera 1989) and integration of this pigment into the eggshell provides additional strength. When a female bird has insufficient calcium to deposit in a shell, protoporphyrin molecules that have a semi-crystalline structure similar to that of eggshells are apparently deposited instead instead of calcium. As a result, the spots occur precisely where the shell is a bit thinner.

Several species of birds have blue eggs, and David Lack (1958) suggested that, in habitats where light levels are low, blue eggs might be cryptic. If true, that could help explain the blue eggs of some open-cup nesting birds that occur in forest habitats such as Wood Thrushes. However, Lack’s hypothesis cannot explain why some birds that nest in cavities, like European Starlings and Eastern Bluebirds, also have blue eggs. One hypothesis is that the blue-green color of eggshells represents a signal of female quality to their mates ( Moreno and Osorno 2003). The pigment responsible for the blue-green color is biliverdin, a substance produced when the hemoglobin of damaged red blood cells is catabolized and also known to have strong antioxidant properties. Antioxidants are important because they can convert free radicals, molecules that can damage DNA, proteins, and other macromolecules, into less reactive substances. Deposition of this pigment in eggshells by laying females may, therefore, signal their capacity to produce antioxidants and control free radicals. Male birds paired to females of such quality that they are able to deposit antioxidants in eggshells rather than retaining them may then expend greater effort in caring for their superior offspring (Kilner 2006). In support of this hypothesis, the provisioning rates of male Pied Flycatchers (Ficedula hypoleuca) and the intensity of the blue coloration of eggs were found to be positively correlated (Moreno et al. 2004). Also, female Eastern Bluebirds in better body condition were found to lay more colorful eggs, supporting the hypothesis that biliverdin pigmentation in eggshells reflects female condition (Siefferman et al. 2006).
Egg-laying:
"Initially the female stood motionless in the nest cup. The first sign of approaching egg- laying was usually intensified breathing, occasionally with rhythmic opening and closing of the bill that pointed either horizontally forwards or more or less upwards. The head was drawn in and the body feathers were somewhat fluffed out; the Coal Tit in addition raised its crown feathers. The tail was kept horizontal or elevated up to about 45 degrees”. Then the tip of the tail started nodding movements synchronously with rhythmic depressions of the rump.These movements which apparently were caused by throes of parturition when the egg traveled down the oviduct, were almost invisible to begin with but gathered in strength and ended with a sudden elevation of the rump that marked the moment of egg-laying. Then the bird “froze” in a motionless posture, termed “recovery phase.” This last rise of the rump clearly indicated that the egg had just been laid.
Duration of egg-laying varies a great deal even within species. The opening and closing of the bill and rhythmic movements of the back and tip of the tail occurs repeatedly for up to 4 minutes in the Prairie Warbler, presumably corresponding to the duration of egg- laying. For 3 eggs of the Goldcrest, only 8-9 seconds elapsed between the first visible sign of pressure and the moment of egg- laying. In tits, this period varied from about 10 to 77 seconds, mostlv 20-30 seconds. The Cuckoo (Cuculus canorth) which is a brood parasite, is known to lay the egg remarkably swift, usually within 10 seconds with a lower limit of only 3-4 seconds. Presumably this short duration is an adaptation to its parasitic behavior."--- From: Haftorn (1996).

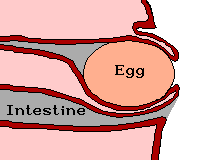
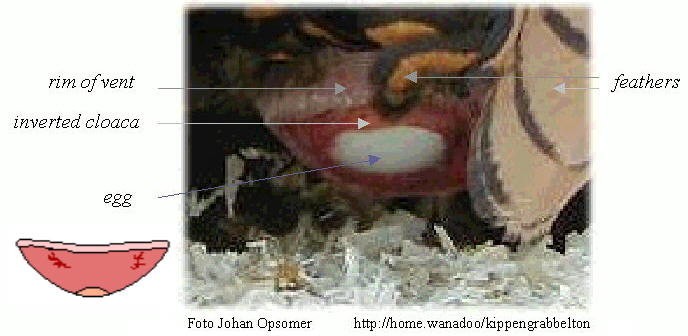
Source: http://www.afn.org/~poultry/egghen.htm
Female birds turns part of the cloaca and the last segment of the oviduct inside out ("like a glove"). The vent is then everted and the egg emerges far outside at the end of the bulge. As a result, the egg does not contact the walls of the cloaca and get contaminated by feces. In addition, the intestine and inner part of the cloaca are kept shut by the emerging egg, and their contents cannot leave when the hen strains to deliver the egg. Therefore, eggs are always clean when laid (van der Molen 2002).
Ostrich lays an egg
Literature Cited:
Akins, C. & M. Burns. 2001. Visual control of sexual behavior. In R. G. Cook (Ed.), Avian visual cognition [On-line]. Available: www.pigeon.psy.tufts.edu/avc/akins/
Birkhead, T. R. and J.-P. Brillard. 2007. Reproductive isolation in birds: postcopulatory prezygotic barriers. Trends in Ecology and Evolution 22: 266-272.Blas, J., L. Perez-Rodriguez, G. R. Bortolotti, J. Vinuela, and T. A. Marchant. 2006. Testosterone increases bioavailability of carotenoids: insights into the honesty of sexual signaling. Proceedings National Academy of Sciences 103: 18633-18637.
Blount, J. D., D. C. Houston, and A. P. Møller. 2000. Why egg yolk is yellow. Trends in Ecology and Evolution 15: 47-49.
Boulinier, H. and V. Staszewski. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends in Ecology and Evolution 23: 282-288.
Brennan, P. L. R., C. J. Clark, and R. O. Prum. 2010. Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia. Proceedings of the Royal Society B 277: 1309-1314.
Brennan, P. L., R. O. Prum, K. G. McCracken, M. D. Sorenson, R. E. Wilson, and T. R. Birkhead. 2007. Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2: e418.
Brown, C.R. and M. B. Brown. 2003. Testis size increases with colony size in cliff swallows. Behavioral Ecology 14:569-575.
Burley, R. W. and D. V. Vadhera. 1989. The avian egg. John Wiley, New York, NY.
Castro, I., E. O. Minot, R. A. Fordham, and T. R. Birkhead. 1996. Polygynandry, face-to-face copulation and sperm competition in the Hihi Notiomystis cincta (Aves: Meliphagidae). Ibis 138: 765-771.
Dzialowskil, E. M. and P. R. Sotherland. 2004. Maternal effects of egg size on emu Dromaius novaehollandiae egg composition and hatchling phenotype. J. Exp. Biol. 207:597-606.
Eising, C. M., W. Müller & T. G.G. Groothuis. 2006. Avian mothers create different phenotypes by hormone deposition in their eggs. Biol. Letters 2: 20-22.
Ellegren, H. 2001. Hens, cocks and avian sex determination: a quest for genes on Z or W? European Molecular Biology Organization Reports 2:192-196.
Ezaz, T., R. Stiglec, F. Veyrunes, and J. A. Marshall Graves. 2006. Relationships between Vertebrate ZW and XY Sex Chromosome Systems. Current Biology 16: R736-R743.
Freedman, S. L., V. G. Akuffo, and M. R. Bakst. 2001. Evidence for the innervation of sperm storage tubules in the oviduct of the turkey (Meleagris gallopavo). Reproduction 121: 809-814.
Gosler, A.G., J. P. Higham, and S. J. Reynolds. 2005. Why are birds’ eggs speckled? Ecology Letters 8: 1105-1113.
Goth, A. and D. T. Booth. 2005. Temperature-dependent sex ratio in a bird. Biology Letters 1: 31-33.
Grindstaff, J. L., E. D. Brodie III, and E. D. Ketterson. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. Royal Soc. B 270: 2309-2319.
Hincke et al. 2012. The Eggshell: Structure, Composition and Mineralization. Frontiers in Bioscience 17:1266–1280.
Kilner, R. M. 2006. The evolution of egg colour and patterning in birds. Biological Reviews 81: 383–406.
Haftorn, S. 1996. Egg-laying behavior in tits. Condor 98:863-865.
King, L.M., J. P. Brillard, W.M. Garrett, M.R. Bakst, and A.M. Donoghue. 2002. Segregation of spermatozoa within sperm storage tubules of fowl and turkey hens. Reproduction 123:79-86.
Lack, D. 1958. The significance of the colour of turdine eggs. Ibis 100: 145-166.
Martin, T. E. 2008. Egg size variation among tropical and temperate songbirds: an embryonic temperature hypothesis. Proceedings of the National Academy of Sciences USA 105: 9268-9271.
Moreno, J. and J. L. Osorno. 2003. Avian egg color and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters 6: 803-806.
Moreno, J., J. L. Osorno, J. Morales, S. Merino, and G. Tomás. 2004. Egg coloration and male parental effort in the Pied Flycatcher Ficedula hypoleuca. Journal of Avian Biology 35:300–304.
Pettingill, O.S., Jr. 1985. Ornithology in Laboratory and Field, Fifth ed. Academic Press, New York, NY.
Pitcher, T. E., P. O. Dunn & L. A. Whittingham. 2005. Sperm competition and the evolution of testes size in birds. Journal of Evolutionary Biology 18: 557-567.
Rosenzweig, M.R., A.L. Leiman and S.M. Breedlove. 1996. Biological Psychology. Sinauer Associates, Sunderland, MA.
Rutstein, A.N., P. J. B. Slater, and J. A. Graves. 2004. Diet quality and resource allocation in the zebra finch. Proc. R. Soc. B (Suppl.) 271: S286-S289.
Saether, S. A., P. Fiske, & J. A. Kalas. 2001. Male mate choice, sexual conflict and strategic allocation of copulations in a lekking bird. Proceedings of the Royal Society B 268: 2097 - 2102.
Samour, Jaime H. 2002. The Reproductive Biology of the Budgerigar (Melopsittacus undulatus): Semen Preservation Techniques and Artificial Insemination Procedures. Journal of Avian Medicine and Surgery 16: 39-49.
Sato, T., Yen-nien Cheng, Xiao-chun Wu, D. K. Zelenitsky, & Yu-fu Hsiao. 2005. A Pair of Shelled Eggs Inside A Female Dinosaur. Science 308:375.
Siefferman, L., K. J. Navara, and G. E. Hill. 2006. Egg coloration is correlated with female condition in Eastern Bluebirds (Sialia sialis). Behavioral Ecology and Sociobiology 59: 651-656.
Sotherland, P. R. and H. Rahn. 1987. On the composition of bird eggs. Condor 89: 48-65.
Stoddard, M. C. 2022. Bird eggs. Current Biology 32: R1126-R1132.
Stoddard et al. 2017. Avian egg shape: Form, function, and evolution. Science 356: 1249–1254.
van der Molen, W. H. 2002. Laying an egg. http://www.afn.org/~poultry/egghen.htm.
Verboven, N., P. Monaghan, D.M. Evans, H. Schwabl, N. Evans, C. Whitelaw, and R.G. Nager. 2003. Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the Lesser Black-backed Gull (Larus fuscus). Proceedings of the Royal Society: Biological Sciences 270: 2223 - 2232
Visser, M. E., L. J. M. Holleman, and S. P. Caro. 2009. Temperature has a causal effect on avian timing of reproduction. Proceedings of the Royal Society B 276: 2323-2331.
Welty, J.C. and L. Baptista. 1988. The life of birds, fourth ed. Saunders College Publishing, New York, NY.
Wingfield, J. C., and T. P. Hahn. 1994. Testosterone and territorial behaviour in sedentary and migratory sparrows: Animal Behaviour 47: 77–89.
Wingfield, J. C., J. D. Jacobs, A. D. Tramontin, N. Perfito, S. Meddle, D. L. Maney, and K. Soma. 2000. Toward and ecological basis of hormone-behavior interactions in reproduction of birds: In K. Wallen and J. Schneider (eds.), Reproduction in context, pp. 85–128. MIT Press, Cambridge.
Winterbotton, M., T. Burke, & T. R. Birkhead. 2001. The phalloid organ, orgasm and sperm competition in a polygandrous bird: the red-billed buffalo weaver. Behavioural Ecology and Sociobiology 50: 474-482.