| BIO 554/754
Ornithology Lecture Notes 3 - Bird Flight II |
 |
| BIO 554/754
Ornithology Lecture Notes 3 - Bird Flight II |
 |
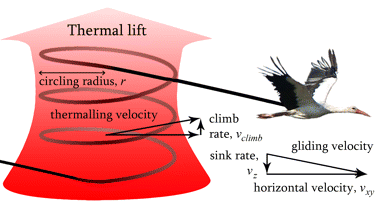
Birds fly in a variety of ways, ranging from gliding to soaring to flapping flight to hovering. Of these, the simplest type of flight is gliding.
Source: Ákos et al. (2008)
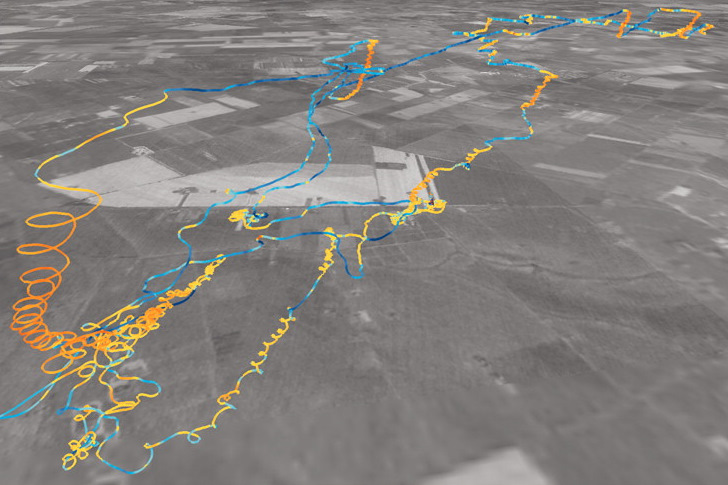
Trajectory or flight path of a Peregrine Falcon superimposed on a black and white satellite map of the area (southeast Hungary).
Color indicates
vertical velocity, with more reddish color indicating climbing within thermals and bluish color indicating sinking
(i.e., periods of gliding
between
thermals) (Source: Ákos et al. 2008).
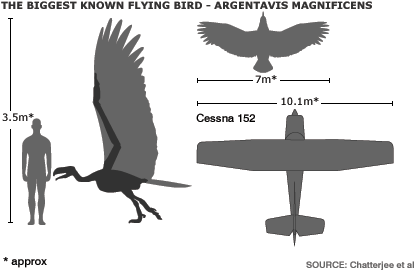
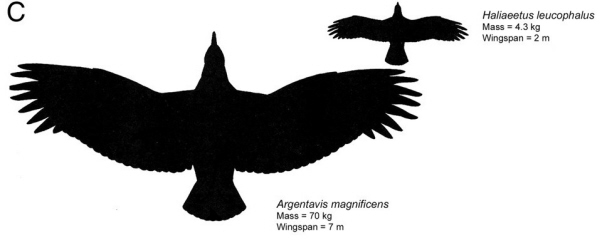
Dorsal wing profile in silhouette of Argentavis is compared for scaling with those of a Bald Eagle.
Argentavis magnificens from the upper Miocene (6 million years ago) of Argentina, with an estimated mass of 70–72 kg and a wingspan of 7 m, was the world's largest known flying bird. Because the fossils of Argentavis are found in the foothills of the Andes to the pampas, it is likely that it used primarily slope soaring over the windward slopes of the Andes and thermal soaring over the open pampas. In slope soaring, a bird flies in a region of rising air caused by upward deflection of wind over a ridge or a cliff. If the sinking speed of the animal is less than the velocity of the rising air, the bird is able to remain airborne indefinitely without flapping its wings. Cranial morphology indicates that Argentavis, like other teratorns, was an active predator rather than a scavenger. It was probably a diurnal predator, dependent on thermals for flight activity for much of the time much as large, broad-winged carnivorous birds we see today. Strong thermals occur by mid-day and disappear in the evening, so thermal soaring for Argentavis would have been possible only between those times. With a skull >55 cm long and 15 cm wide, Argentavis was capable of catching sizeable prey with its formidable beak -- From: Chatterjee et al. (2007).

Wandering Albatross
© Paul Ward and Cool
Antarctica
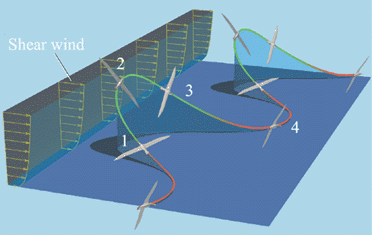 |
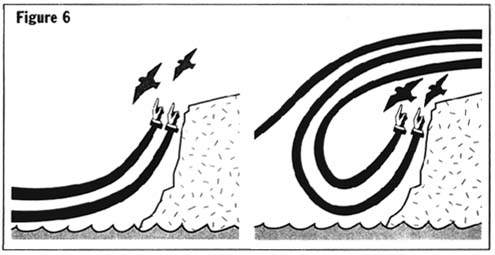
Dynamic soaring -- Albatrosses perform a fascinating and complicated flight maneuver called dynamic soaring, in which energy can be extracted from horizontally moving air and transferred to the bird so that an energy gain is achieved which enables it to fly continuously without flapping. Dynamic soaring is possible when the wind speed changes with altitude. This type of wind, which is called shear flow, exists in the boundary layer above the ocean surface in areas in which albatrosses are found. Dynamic soaring consists of periodically repeated cycles, with one cycle illustrated to the left: 1 - climb (windward flight); 2 - upper curve (change of flight direction to leeward); 3 - descent (leeward flight); & 4 - lower curve (change of flight direction to windward) (Sachs 2005). |
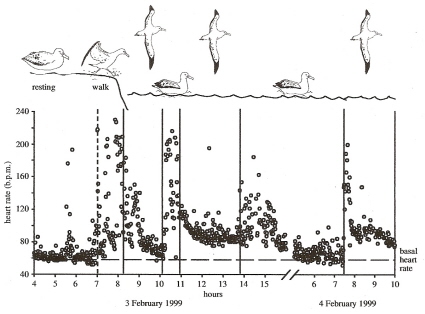
Dynamic soaring is energetically efficient. The heart rate of a Wandering Albatross was recorded over a two-day period
and its heart rate was just above resting rates when soaring, suggesting that dynamic soaring requires little more energy than
resting on land (Weimerskirch et al. 2000).
Procellariiform birds: the ‘tube knows’ air speed? -- Albatrosses and smaller Procellariiform birds like petrels and shearwaters can travel long distances over the ocean by dynamic soaring. This method of soaring requires that these birds be able to detect variation in wind speed at various distances above the ocean surface. Pennycuick (2002) proposed that albatrosses and their relatives can use their tubenoses as a pitot tube to very accurately determine air speed. Pitot tubes on airplanes have two holes, one pointing forward (in the direction of the plane is flying) to measure what is called the stagnation (or pitot) pressure, and a side hole that measures static pressure (the ambient pressure of the surrounding air). The difference between the stagnation pressure and static pressure is called the dynamic pressure, which can be used to determine a plane’s airspeed. For example, in the diagram below, as a plane increases its airspeed, the pressure generated at the stagnation point will increase relative to the static pressure and the fluid in the differential manometer will be forced downward, out of the manometer, and upward in the tube leading out of the manometer. Pitot tubes are calibrated so that dynamic pressure readings are ‘translated’ into airspeed readings.
The tubenoses of albatrosses and other Procellariiform birds resemble pitot tubes and may function in the same way. The forward-pointing nostrils or tubenoses (pointing in the direction the birds fly), that could measure stagnation or pitot pressure, lead into nasal chambers also connected to the mouth, or oral, cavity, where pressure would correspond to static pressure. Mangold (1946) identified an expandable pocket or capsule on either side of the nasal septum of petrels and proposed that these were sense organs that could measure dynamic pressure, the difference between stagnation and static pressure, and provide information about airspeed. Additional study is needed to test Pennycuick’s (2002) and Mangold’s (1946) hypothesis.
|
 during such flight, different parts of a wing have different functions:
during such flight, different parts of a wing have different functions:
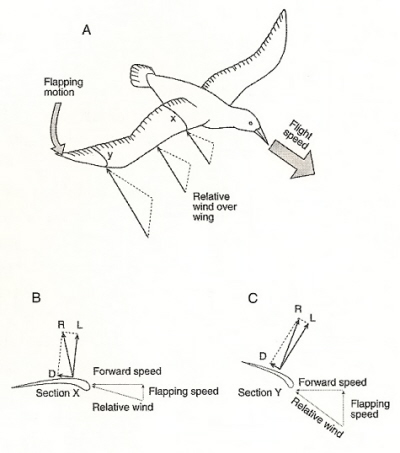
Different forces along a flapping wing. (A) There is little vertical movement of the wing close to the bird's body, but
the distal portion of the wing
is angled downward (with the leading edge lower than the trailing edge) and air moving past the distal wing
is moving faster, and at a different angle, because of the wing’s flapping motion. (B) At cross-section X, the lift is almost vertical. (C) At
cross-section Y, because of the angle, the lift force is tilted forward and produces forward thrust. D = drag force, L = lift force, R = resultant force
(From: Alexander 2002; Fig. 4.6).

These images, taken from a high-speed recording of a
cockatiel flying at 1 meter/sec, show the tip-reversal upstroke.
In the first frame, the wing has already reversed
direction
and the humerus has been elevated. In the second frame,
the primary feathers have rotated slightly to create
gaps between successive feathers. Between the second and third frames,
the rotated primaries sweep upward as the wrist joint
extends. By the third frame, the primaries have been rotated back into
their standard orientation and the wing has begun to
move forward as well as upward (Hedrick et al. 2004).
A Black-billed Magpie flying in a windtunnel at two different speeds
wearing custom respirometry masks.
Cockatiel flying in a wind tunnel
Gull in slow motion
Rock Pigeons in flight
Birds taking off - slow motion
Most species of birds do not flap their wings continuously during flight. Rather, they exhibit one of two intermittent flight patterns: flap-gliding and flap-bounding. Mathematical models predict that flap-bounding is energetically cheaper than continuous flapping flight at high speeds, while flap-gliding is more efficient than continuous flapping at low speeds. However, few species of bird exhibit both types of intermittent flight, so flap-bounding may be a compromise between the need to maintain muscle contractions at an optimal velocity and the need to vary power output and flight speed. In addition, the primary flight muscle, the pectoralis, of many small birds is composed of a single muscle fiber type, further limiting the range of useful strain rates for these species. Thus, a "fixed-gear hypothesis" suggests that the only economical method for small birds to vary power output is to intermittently bound. However, investigators at the Flight Laboratory at the University of Montana have found that some small birds, such as Budgerigars and European Starlings, do exhibit both types of intermittent flight, with flap-gliding being used at lower speeds, and flap-bounding at higher speeds. This suggests that some small birds are capable of optimizing their flight styles despite the theoretical constraints of their muscle composition.

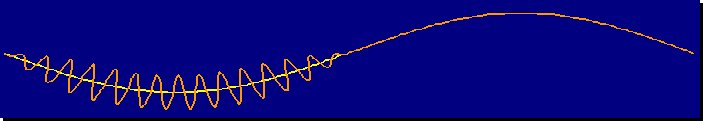
Flap-gliding flight path (left) and flap-bounding flight path (right)
Source: http://www.biology.leeds.ac.uk/staff/jmvr/Flight/PWV/index1.htm
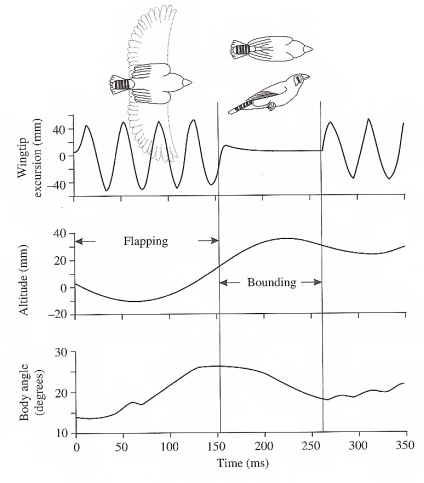
Timing of a flap-bounding cycle relative to wing movements, altitude, and body angle (relative to horizontal) for a
Zebra Finch flying in a wind tunnel (Videler 2005, based on Tobalske et al. 1999).
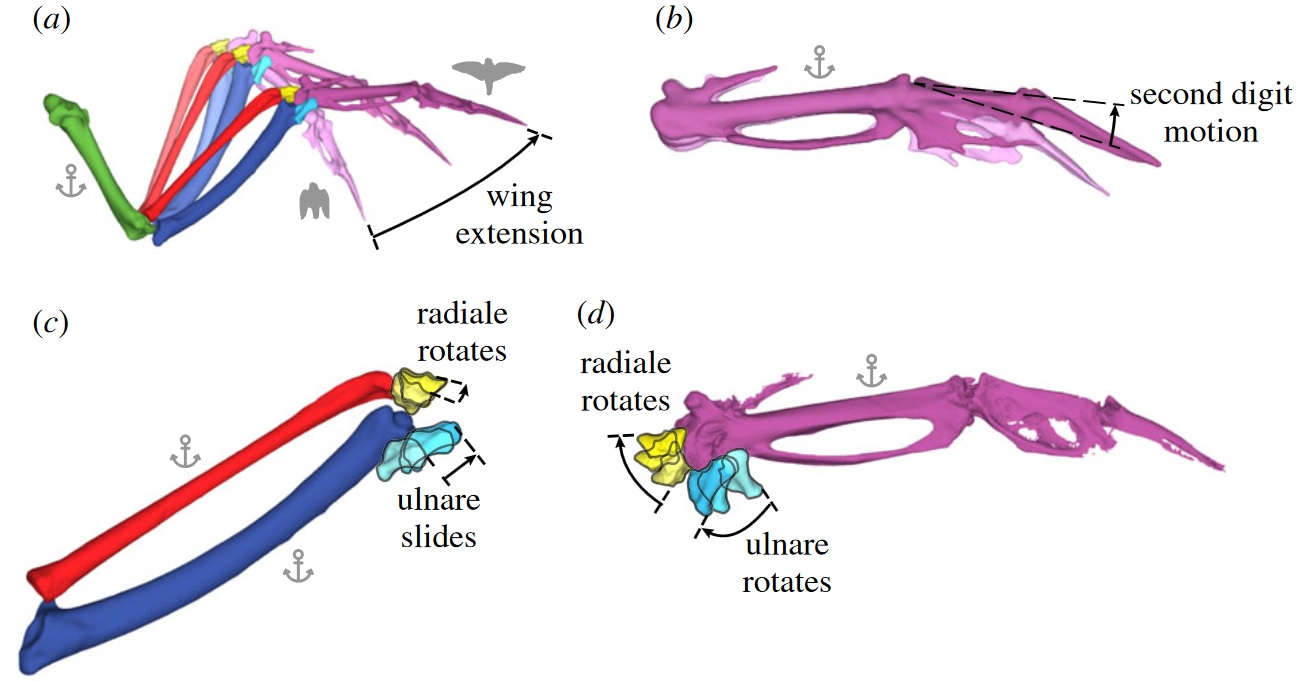
During wing flexion and extension, the second digit can move independently while the wrist bones rotate and slide in concert with the connecting bones. (a) As the wing extends, the elbow and wrist move in a coordinated fashion.
(b) The second digit can move independently up to 30 degrees, allowing fine control of wingtip motion. (c) Relative to the lower radius and ulna, the ulnare slides primarily along the ulna while the radiale remains relatively stationary.
(d) Both the radiale and ulnare primarily rotate about the head of the carpometacarpus. Anchors indicate stationary bones to show relative motion of other bones (Figure from Stowers et al. 2017).
Source: http://biology.umt.edu/flightlab/Intermittent.htm
As flight speed increased in a wind tunnel, budgerigars that
exhibited
intermittent flight at all speeds tended to flex their
wings during intermittent non-flapping periods, apparently in response
to increased profile drag (Tobalske and Dial 1994).
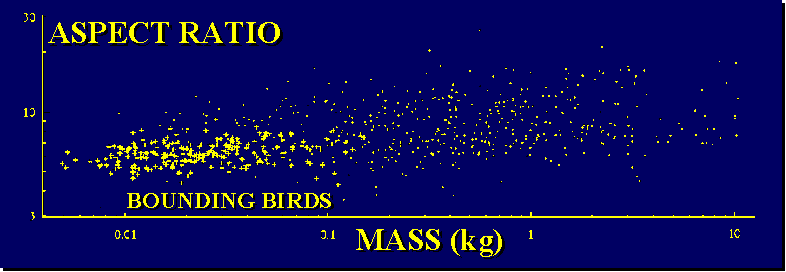
Bounding flight is not seen in birds of greater than 300 grams, thus likely to be constrained by size. The small birds which most frequently utilize bounding tend to have short rounded wings (low aspect ratio), poorly suited for gliding, making undulating flight less aerodynamically attractive.
(Source: www.biology.leeds.ac.uk/staff/jmvr/Flight/PWV/index1.htm)

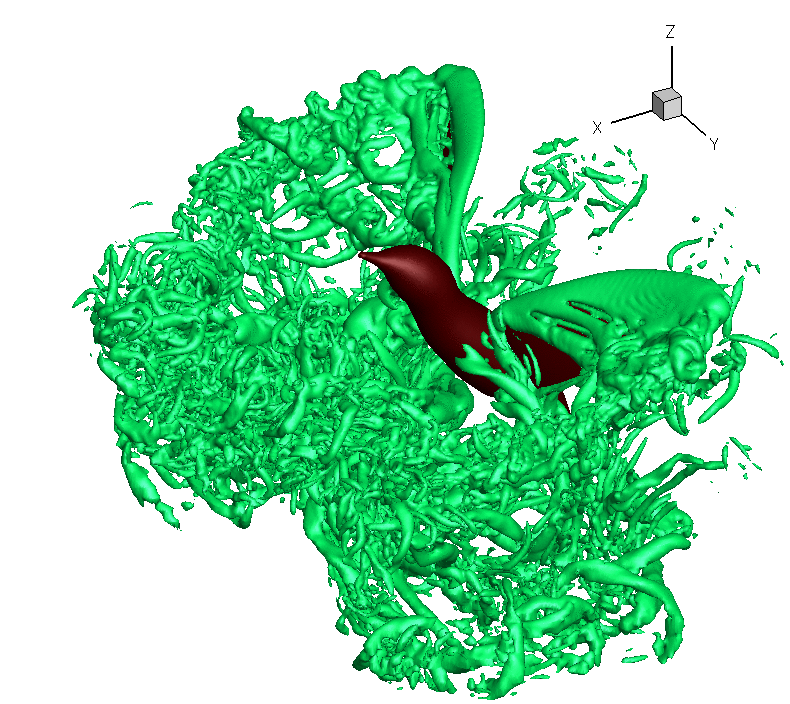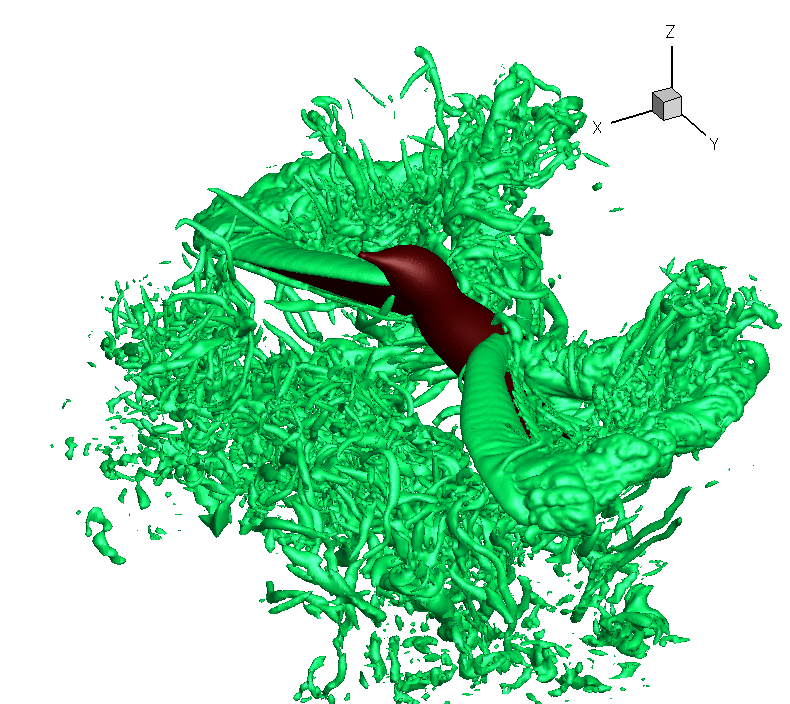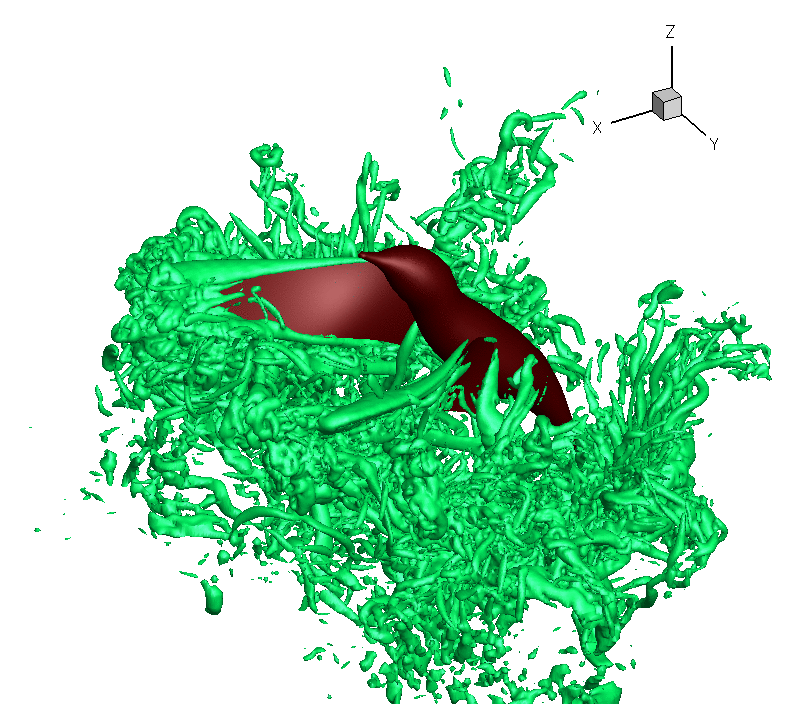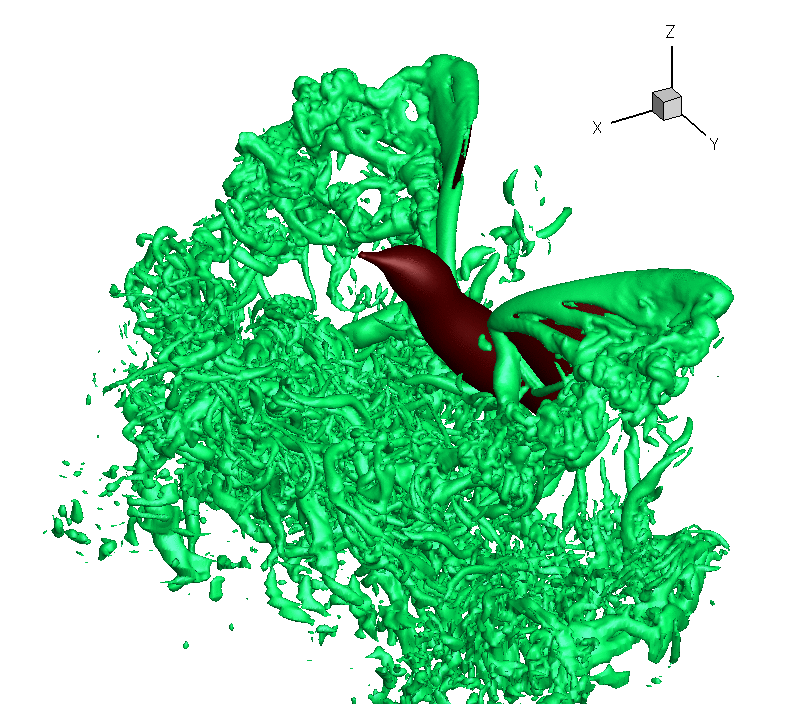
Turbulence created as a Ruby-throated Hummingbird hovers with wings beating about 40 times per second. The hummingbird downstroke generates about 2.5 times as
much vertical force as the upstroke. Associated with lift production is
the similar power imbalance between the two half strokes. Analysis indicates that in addition to the angle of
attack, wing velocity and
surface
area,
drag-based force
and wing–wake interaction also
contribute significantly to the lift asymmetry (From: Song et al. 2014). Also, check this
link - Science graphic of the week: hummingbird wing aerodynamics.

Source: http://www.njsouth.com/leamingsrun.htm
Female Ruby-throated Hummingbird (about 45 wingbeats/second)
Broad-tailed Hummingbirds (slow motion)
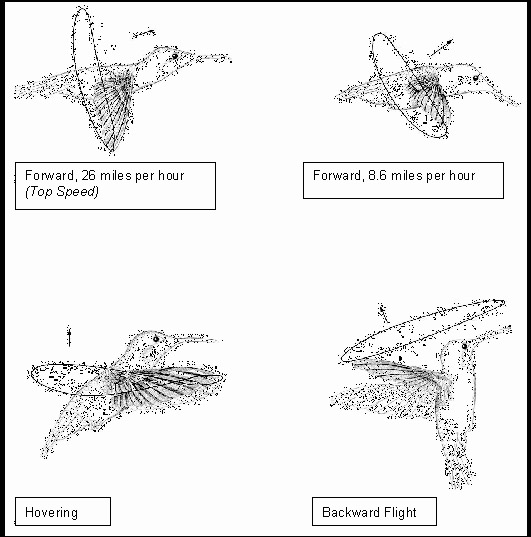
Source: http://www.ae.utexas.edu/design/humm_mav/theory.html
Loss of gluconeogenic muscle enzyme contributed to adaptive metabolic traits in hummingbirds -- Hummingbirds possess distinct metabolic adaptations to fuel their energy-demanding hovering flight, but the underlying genomic changes are largely unknown. Here, we generated a chromosome-level genome assembly of the long-tailed hermit and screened for genes that have been specifically inactivated in the ancestral hummingbird lineage. We discovered that FBP2 (fructose-bisphosphatase 2), which encodes a gluconeogenic muscle enzyme, was lost during a time period when hovering flight evolved. We show that FBP2 knockdown in an avian muscle cell line up-regulates glycolysis and enhances mitochondrial respiration, coincident with an increased mitochondria number. Furthermore, genes involved in mitochondrial respiration and organization have up-regulated expression in hummingbird flight muscle. Together, these results suggest that FBP2 loss was likely a key step in the evolution of metabolic muscle adaptations required for true hovering flight (Osipova et al. 2023). |
|
||
| Does a hummingbird fly like an insect or a
bird? A
bit like both. Many experts had argued that hummingbirds' skill
at
hovering, of which insects are the undisputed masters, means that the
two
groups may stay aloft in the same way: by generating lift from a wing's
upstroke as well as the down. This turns out to be only partially true.
Other birds get all of their lift from the downstroke (during slow flight and when hovering; not during faster flight), and insects
manage
to get equal lift from both up and down beats (check this short video), but hummingbirds lie
somewhere in between and gett about 75% of their lift from the
downstroke
and 25% from the upstroke.
To determine this, Warrick
et al. (2005)
trained Rufous Hummingbirds (Selasphorus rufus) to hover in
place
while feeding from a syringe filled with sugar solution and looked at
the
swirls of air left in their wake. They filled the air with a mist of
microscopic
olive-oil droplets, and shone a sheet of laser light in various
orientations
through the air around the birds to catch two-dimensional images of air
currents. A couple of quick photographs taken a quarter-second apart
caught
the oil droplets in the act of swirling around a wing. Although
hummingbirds
do flap their wings up and down in relation to their body, they tend to
hold their bodies upright so that their wings flap sideways in the air.
To gain lift with each stroke the birds partially invert their wings,
so
that the aerofoil points in the right direction. Their flight looks a
little
like the arm and hand movements used by a swimmer when treading water,
albeit it at a much faster pace. |
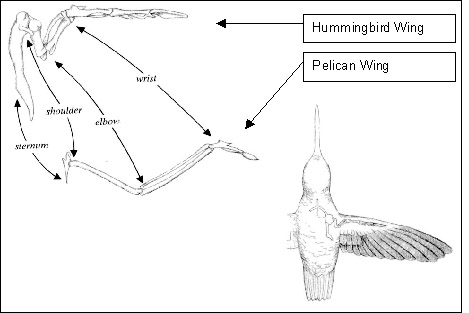
Hummingbirds flying in the rain -- Flight in rain represents a greater challenge for smaller animals because the relative effects of water loading and drop impact are greater at reduced scales given the increased ratios of surface area to mass. Nevertheless, it is well known that small volant taxa such as hummingbirds can continue foraging even in extreme precipitation. Ortega-Jimenez and Dudley (2012) evaluated the effect of four rain intensities (i.e. zero, light, moderate and heavy) on the hovering performance of Anna's Hummingbirds (Calypte anna) under laboratory conditions. Light-to-moderate rain had only a marginal effect on flight kinematics; wingbeat frequency of individuals in moderate rain was reduced by 7% relative to control conditions. By contrast, birds hovering in heavy rain adopted more horizontal body and tail positions, a position that may reduce the number of drops hitting a bird's wings and keep it more stable in the air. In heavy rain, hummingbirds also increased wingbeat frequency substantially, while reducing stroke amplitude when compared with control conditions. The ratio between peak forces produced by single drops on a wing and on a solid surface suggests that feathers can absorb associated impact forces by up to approximately 50%. Remarkably, hummingbirds hovered well even under heavy precipitation (i.e., 270 mm h−1) with no apparent loss of control, although mechanical power output assuming perfect and zero storage of elastic energy was estimated to be about 9% and 57%t higher, respectively, compared with normal hovering.
Links:
Secret of hummingbirds' ability to fly in the rain
| Hovering is hard work for most birds - Ever seen a songbird hover over a crowded feeding station, waiting for a perch to open up so it can land and eat? Looks like hard work, doesn't it? It is, which is why hovering is something most birds don't like to do -- or can't do -- for very long. Dial et al. (1997) surgically implanted strain gauges in the wings of three Black-billed Magpies. The devices measured the force exerted by the main flapping muscle with each wing beat. The birds then flew in a wind tunnel at a range of speeds. The strain gauge allowed the scientists to calculate the power (the amount of work done per unit time) required to maintain a given speed. Hovering took nearly twice as much power as flying at average speed, the researchers found. Even when the magpies flew at top speed, they expended far less power than they did when they hovered. Evidence suggested that when they hovered, the birds were working at their physical limits. Their wing muscles appeared to be employing anaerobic metabolism, a source of energy that can't be sustained for long. There are clearly exceptions to this. Hummingbirds, the authors note, have an unusual shoulder design that allows them to generate lift on both down-beat and up-beat. But birds with a body design similar to magpies are likely to have strict limits on their abilities to fly standing still. |
Kestrel hovering
Formation Flying
Some birds, like geese & cranes, are often observed flying in V-formation. The reason is wingtip vortices. The birds take advantage of the upwind side of the vortex shedding off the bird in front of them. This updraft actually lifts the bird up, making the flight a little easier.
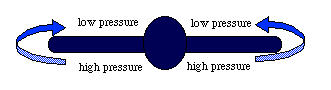 |
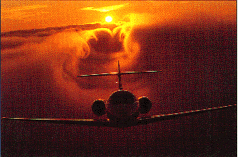 |
Air moves from the area of high pressure (under the wing) to the area of low pressure (top of the wing) at the wing tips. This is nicely illustrated in the photo of the plane passing through clouds. Birds flying in V-formation use these vortices of rising air.
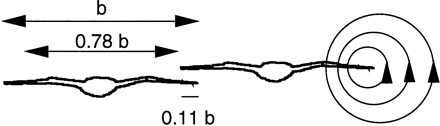
A vortex is formed in the wake of each wingtip, creating downflow behind the wing and uplift outside
the wake, as indicated
at the tip of the right wing of the right-hand bird. A trailing bird can take energetic advantage
of this uplift by flying at a suitably lateral position relative to the bird ahead. Theory suggests that the optimal wingtip
overlap for the trailing bird is about one tenth of the wingspan b. A distance of about 0.78b separates the centers
of the two trailing vortices from a bird or aircraft (Andersson and Wallander 2004).

See "Mystery
of bird 'V' formation solved" (BBC News)
Seminar - Bird flight and cooperative dynamics by Steve Portugal
Winged Migration
Flying in a flock comes at a cost -- Flying birds often form flocks, with social, navigational, and anti-predator implications. Further, flying in a flock can result in aerodynamic benefits, thus reducing power requirements, as demonstrated by a reduction in heart rate and wingbeat frequency in pelicans flying in a V-formation. But how general is an aerodynamic power reduction due to group-flight? V-formation flocks are limited to moderately steady flight in relatively large birds, and may represent a special case. What are the aerodynamic consequences of flying in the more usual ‘cluster’ flock? Usherwood et al. (2011) used data from back-mounted Global Positioning System (GPS) and inertial sensors to show that pigeons (1) maintain powered, banked turns like aircraft, imposing dorsal accelerations of up to 2 g, effectively doubling body weight and quadrupling induced power requirements, (2) increase flap frequency with increases in all conventional aerodynamic power requirements, and (3) increase flap frequency when flying near, particularly behind, other birds. Therefore, unlike V-formation pelicans, pigeons do not gain an aerodynamic advantage from flying in a flock. Indeed, the increased flap frequency, whether due to direct aerodynamic interactions or requirements for increased stability or control, suggests a considerable energetic cost to flight in a tight cluster.
Food
and formation help birds fly efficiently. Swimming after a heavy meal may not be wise - but flying is another
matter.
Birds fly more efficiently when loaded with food, recent research
suggests,
helping to explain how they can migrate thousands of kilometres without
stopping (Kvist et al. 2001). And a second study has confirmed the
century-old
suspicion that birds fly in a V formation to save substantial amounts of energy (Weimerskirch et al. 2001). Anders Kvist at Lund
University
in Sweden and his colleagues looked at flying efficiency in Red Knots
(shown
to the right), small waders that double in size for their annual
migration
from Siberia to Africa. Fully fed, Red Knots flying in a wind tunnel
for
6-10 hours extracted significantly more power from each unit of food.
This
might help to explain why birds often make long non-stop flights even
when
they don't have to cross an ocean or desert, says Kvist. "Since
efficiency
increases when the birds are heavy, it might not be as bad to make long
flights as people thought." The research flies in the face of computer
predictions that birds are less efficient when full. Says bird
aerodynamics
specialist Jeremy Rayner of the University of Leeds: "It's a
major
advance, because it has disproved something we've held on to for a long
time." The finding is "extremely unexpected", agrees John
Speakman
who works on animal energy use at the University of Aberdeen. "This
changes
our whole view of migrational strategies in terms of how much fat birds
should deposit to cross, say, the Sahara Desert." Understanding the
relationship
between food and flight might help ecologists to measure the impact of
habitat change on migratory birds, Speakman says. "If you're deciding
whether
to flood an estuary, for example, this could help you make more
sensible
predictions about how it will affect birds that use the estuary as a
stopover." It is unclear how birds increase their efficiency when migrating, Kvist
says. Puzzlingly, they don't adopt the most economical strategy at all
times. Kvist speculates that when birds are breeding they may keep
reserves
of strength for sudden manoeuvres such as speeding up or swerving to
avoid
a predator. amounts of energy (Weimerskirch et al. 2001). Anders Kvist at Lund
University
in Sweden and his colleagues looked at flying efficiency in Red Knots
(shown
to the right), small waders that double in size for their annual
migration
from Siberia to Africa. Fully fed, Red Knots flying in a wind tunnel
for
6-10 hours extracted significantly more power from each unit of food.
This
might help to explain why birds often make long non-stop flights even
when
they don't have to cross an ocean or desert, says Kvist. "Since
efficiency
increases when the birds are heavy, it might not be as bad to make long
flights as people thought." The research flies in the face of computer
predictions that birds are less efficient when full. Says bird
aerodynamics
specialist Jeremy Rayner of the University of Leeds: "It's a
major
advance, because it has disproved something we've held on to for a long
time." The finding is "extremely unexpected", agrees John
Speakman
who works on animal energy use at the University of Aberdeen. "This
changes
our whole view of migrational strategies in terms of how much fat birds
should deposit to cross, say, the Sahara Desert." Understanding the
relationship
between food and flight might help ecologists to measure the impact of
habitat change on migratory birds, Speakman says. "If you're deciding
whether
to flood an estuary, for example, this could help you make more
sensible
predictions about how it will affect birds that use the estuary as a
stopover." It is unclear how birds increase their efficiency when migrating, Kvist
says. Puzzlingly, they don't adopt the most economical strategy at all
times. Kvist speculates that when birds are breeding they may keep
reserves
of strength for sudden manoeuvres such as speeding up or swerving to
avoid
a predator. Birds also conserve fuel by flying in V formations. By measuring heart rates, researchers in France now have proof that pelicans use 11-14% less energy flying together, even when they are not perfectly positioned to take advantage of the wake from those in front of them. Configured flight may create a stream of air that allows birds to glide longer, suggests Henri Weimerskirch, the biologist at the National Centre of Scientific Research at Villiers en Bois, who led the study. "If you look closely, you see that the birds at the back are gliding more than the leader." People have been asking whether V formations are more efficient for more than 100 years, Speakman says, but no one had measured energy savings before. "They took a century-old problem and went to the heart of it," he says. ---- Written by Erica Klarreich. |
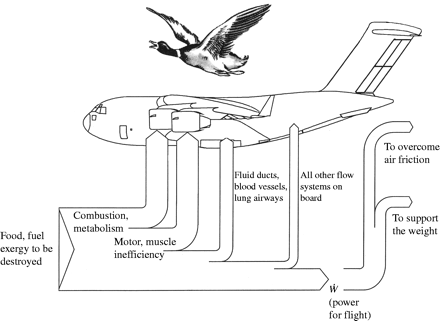
Flight Metabolism
All birds have high metabolic rates, and flying birds have even higher rates. The metabolic cost of flight depends on the type of flight (gliding, soaring, flapping, or hovering), wing shape, and speed. Of course, flapping flight and hovering are the most costly types of flight. Laboratory studies of birds trained to fly in wind tunnels (like the one below) indicate that the metabolic 'cost' of flapping flight can be anywhere from about 7 to 15 times a bird's basal metabolic rate.
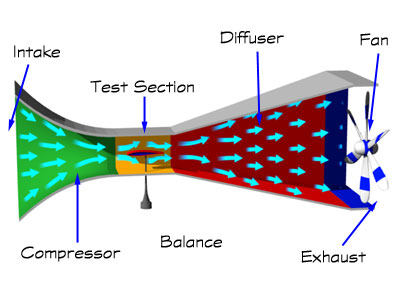
Source: http://www.swe.org/iac/LP/wind_03.html
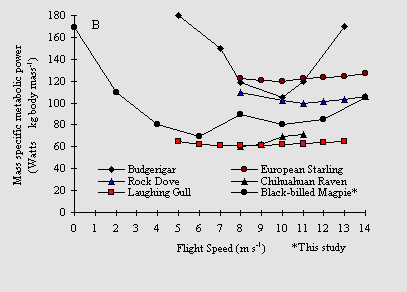
Speed influences the cost of flight, with low speed flight (such as when taking off or landing) requiring more energy. Some information also suggests that bird's flying at maximum speeds also use more energy than at 'medium' speeds. For example, in the graph below, note that European Starlings use much more energy at low speeds (0 - 2 meters/second) than at higher speeds. The relationship between flight speed and energy consumption is also very apparent for Budgerigars (below). Low speed flight is more costly because there is more drag (induced drag). At low speeds, airflow over wings is relatively slow and, to maximize lift, birds must maximize the angle of attack and flap their wings fast to increase air speed and this requires lots of power. High speed flapping flight (as illustrated for Budgerigars and European Starlings below) is more costly because, at greater speeds, friction drag and parasite drag increase, requiring an increase in wing-beat frequency and/or an increase in the proportion of muscle cells (in the pectoralis muscles) contracting. The graphs below clearly reveals that flight is most efficient at 'medium' speed.
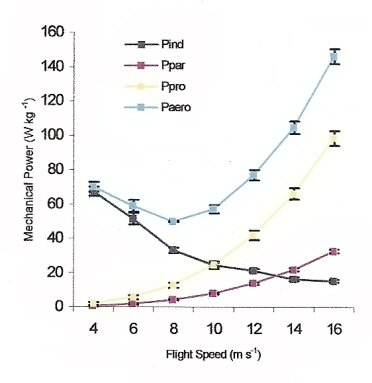
Total mechanical (aerodynamic; Paero) power output during flapping flight
at different flight speeds in Cockatiels (Nymphicus hollandicus). Data represents means ± SE.
Pind = induced power, Ppar = parasite power, and Ppro = profile (friction) power (Brighton 2007).
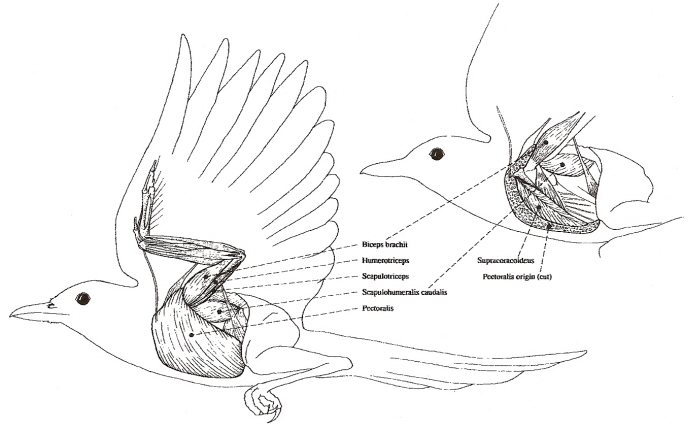
Superficial (left) and deep (right) flight muscles of a Black-billed Magpie (Tobalske et al. 1997).
A 10,000 km non-stop flight -- Four Bar-tailed Godwits (Limosa lapponica) flew their way into the record books with nonstop flights of more than 10,000 km from New Zealand to the Yellow Sea. The godwits, tracked by satellite transmitters in March 2007, did not stop to eat or drink on the first leg of their northern migration that ends in Alaska in May. Phil Battley, an ecologist at Massey University, said it had been suspected that the birds could fly such distances but now it had been proved. No other animal has shown such endurance, he said. The female godwits took 6 to 7 days to cover the route, flying at altitudes up to 2 km and at an average speed of 56 km/h. When the godwits leave New Zealand, they are clinically obese, but lose about half their body weight during each portion of their migratory flight. After arriving in the tidal flats of the Yellow Sea, off China and South Korea, they stay for a month or two to refuel. Battley said "It's the equivalent of riding the Tour de France but keeping it up for a week nonstop." |
|
Birds, of course, get around in ways other than flying. In fact,
some
birds are flightless and depend entirely on walking, running, or 
swimming to get from place to place. Some birds spend most of their time on or in water. Birds have special adaptations of the legs, feet, & wings for terrestrial and aquatic (swimming and diving) locomotion.
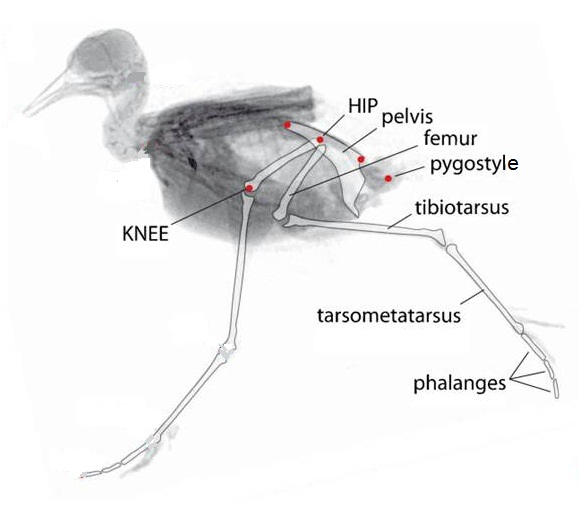
With short, light tails (due to reduction in the number of caudal vertebrae) and large flight muscles, natural selection has favored
positioning of bird feet under a more cranial positioned center of mass. This is achieved by a subhorizontal orientation of the femur
and, when walking
and running,
the knee acting as the main fulcrum near the bird's center of mass (From: Nyakatura et al. 2012).
Western Grebes
Running shorebirds
Waddling makes most of penguin's short legs - It may not be graceful, but the penguin's waddle makes perfect sense to scientists, who found that the bird's side-to-side gait conserves energy. University of California researchers found that the gait works like a pendulum, with energy stored at the end of each swing for the bird's next step. "Our findings indicate that walking is expensive for penguins not because of their waddling, but because they have such short legs that require their leg muscles to generate force very quickly when they walk," said Timothy Griffin, a UC Berkeley graduate student. Griffin and Kram (2000) decided to study penguins because they seem to be doing everything wrong. An earlier study showed penguins were burning twice as many calories when walking as other animals of similar size. But researchers found the problem was the penguins' legs, not their jerky side-to-side movements. The Emperor penguins studied at San Diego's Sea World, for instance, were at least 3 feet tall but had legs only about 10 inches long. Penguins burn about the same amount of calories as animals with similar leg lengths, Griffin said. The researchers coaxed penguins across a force platform -- "kind of a fancy bathroom scale," says Griffin -- with bits of fish. Using scale measurements and videos, the scientists measured the side-to-side and fore-and-aft forces the penguins exert while walking, as well as the vertical forces supporting their weight. The five penguins studied had a walking speed of about 1.5 feet per second. The percentage of energy retained during two steps is called the recovery rate. Humans have a recovery rate of about 65 percent. The penguins studied by Griffin and Kram had an impressive recovery rate of up to 80 percent.

Head movements in walking Whooping Cranes.
(A) One frame of a video recording of a walking crane, showing method
of measurement of head, body, and leg position. The head is fit with a graphical model of the eye and bill, the body with a circle scaled to
head and leg size and centered over the pelvis of the bird, and each lower leg with a line segment extending from the ankle to the foot
(green, right leg; red, left leg). (B) One sequence of measurements, at intervals of 33 ms, of a spontaneously foraging Whooping Crane
through several complete stepping cycles. The bird walked at an average speed of about 0.46ms–1. During this sequence, the right
foot completed nearly 3 steps and the left foot, about 2.5 steps. The head was stabilized throughout most of each foot’s step, with its
positions at each of these times indicated by the arrows. (Watch a crane walk, click here!; video by Thomas Cronin).
Avian head bobbing -- Many species of birds move their heads forward through a series of successive, fixed positions when walking. This unique ‘head-bobbing’ behavior stabilizes visual fields during body movement, preventing motion blur of the retinal image. Gaze stabilization could be required for successful visual search, particularly for moving objects, but the time available for stabilization varies with walking speed. No direct evidence has been published showing that birds favor the stabilization phase while foraging either for moving or immobile food. Cronin et al. (2005) examined head-bobbing behavior in foraging Whooping Cranes (Grus americana) as they searched the ground for food, and found that they walk at speeds that allow the head to be immobilized at least 50% of the time. The stable phase of bird head-bobbing movements is particularly interesting because the behavior, unique to birds, clearly contributes to visual gaze stabilization. Pigeons head-bob when landing, and herons stabilize their heads rigidly when walking or when their perch moves, almost certainly for visual function. Head movements nevertheless play essential roles in vision, giving visual cues for distances and relative locations of objects, providing an opportunity for changes in head angle, and permitting birds to fixate new objects of visual interest.

White-breasted Nuthatch
Source: http://animalpicturesarchive.com/animal/APAsrch3.cgi?qt=nuthatch
Black-and-white Warbler
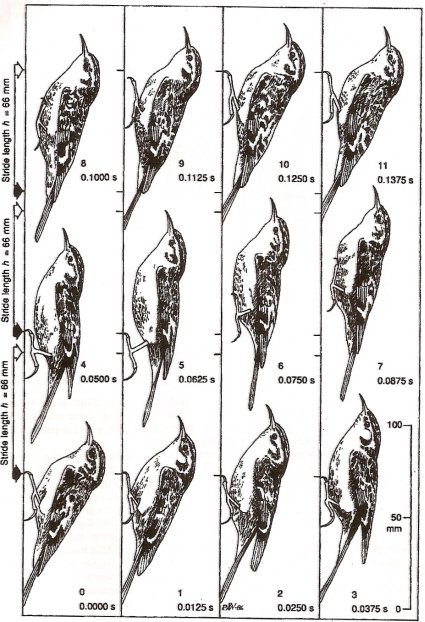
A complete 'hop' of a treecreeper climbing upwards on a vertical trunk. The sequence is from lower left to upper right.
Birds adapted for climbing, like woodpeckers and treecreepers, have sharply recurved claws and toes, sometimes relatively long, that can be spread apart to help firmly grip the substrate (typically tree bark). Other adaptations for climbing differ with foraging habits. Climbers that typically move up trees, like woodpeckers (Picidae) and treecreepers (Certhidae), have relatively short legs (particularly the tibotarsus) that keep their center of mass close to the substrate and a long, stiff tail that provides support against the force of gravity. As woodpeckers and treecreepers move up a tree, they ‘hop’ upward and inward (to counteract the force of gravity that tends to pull them away from a vertical tree trunk), moving both feet in unison. Tail support provides two advantages: (1) the long tail creates a long baseline between the points of attachment (feet and tail) and, the longer this baseline, the smaller the horizontal force between feet and bark against which the bird must work when pulling itself towards the trunk when hopping upward, and (2), when not moving, the tail, rather than the leg muscles, supports part or all of the bird’s weight (Norberg 1981).
Climbing birds that use their tail for support almost always move upwards when foraging. Foraging woodpeckers and treecreepers approaching the top of one tree, typically fly downward to a lower position on another tree then again climb upwards and, when approaching the top, repeat the process. Not only does such a foraging strategy make sense energetically (because flying downward is less costly), but attempting to move downward when foraging would create at least three problems (Norberg 1981): (1) difficulty in seeing where to grasp the bark after a hop, (2) the stiff tail could get caught on the irregular surface of the bark, and (3) potential prey would be alerted to the presence of a possible predator before the bird could get in a position to capture them.
Nuthatches (Sittidae) are adapted for climbing downward as well as upwards. Their relatively short tails are not used for support and, rather than hopping, nuthatches walk up and down tree trunks and branches with alternating leg movements. Nuthatches have relatively long legs (particularly the tibiotarsus), allowing a relatively long baseline between the feet and reducing the horizontal force between feet and bark and the energetic cost of locomotion (Norberg 1981).

Source: http://www.oaklandzoo.org/atoz/video22.html
Wood Duck
A swimming and diving Anhinga
Common Loon
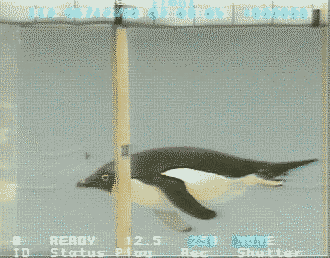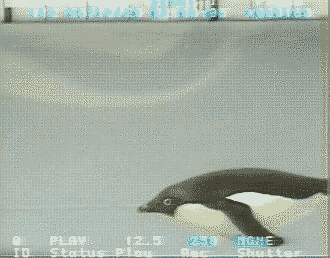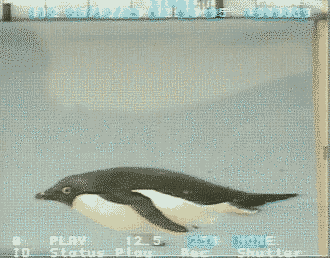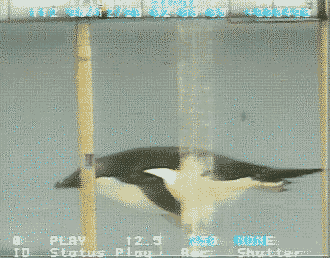
Source: http://www.bionik.tu-berlin.de/intseit2/xs2pinfi.html
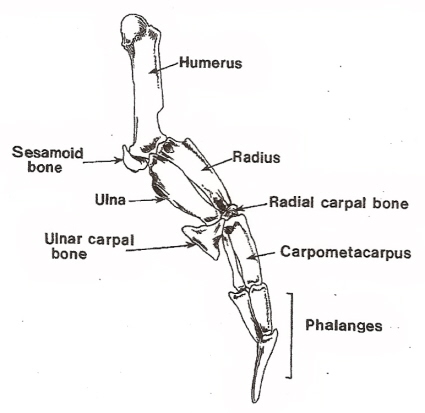
Wing bones of a Jackass Penguin (Spheniscus demersus).
For wing-propelled swimming underwater, the ‘paddle’ must be highly mobile at the shoulder
(humerus-pectoral girdle articulation), but the remaining joints need to be relatively fixed to minimize
the
muscle contraction
needed to maintain the proper position (Louw 1992).
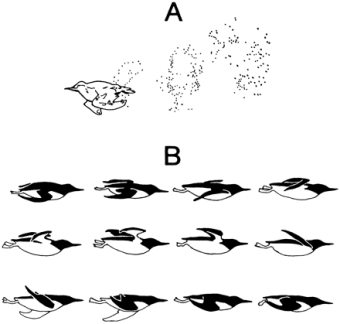
Bubbles in the wake of a Pigeon Guillemot (Cepphus columba) swimming horizontally underwater,
indicating patterns of intermittent thrust mainly on the downstroke. B) Wing positions
during horizontal
swimming by a Common Murre, as drawn from films taken at 32 frames/sec. Sequence is from
left to right and top row to bottom row. Angle of attack of the wings suggests substantial lift during the upstroke
(From: Lovvorn 2001).
Guillemots diving
Rapid ascent of Emperor Penguins -- To jump out of water onto sea ice, Emperor Penguins must achieve sufficient underwater speed to overcome the influence of gravity when they leave the water. The relevant combination of density and kinematic viscosity of air is much lower than for water. Injection of air into boundary layers (air lubrication, i.e., an air film separates the water from the surface of a structure or a bird thus reducing friction) has been used by engineers to speed movement of vehicles (ships, torpedoes) through sea water. Based on analysis of published and unpublished underwater film, Davenport et al. (2011) hypothesized that free-ranging Emperor Penguins employ air lubrication in achieving high, probably maximal, underwater speeds (mean = 5.3 meters/sec), prior to jumps. Penguins dive to 15 to 20 meters with air in their plumage and that compressed air is released as the birds subsequently ascend while maintaining depressed feathers. Fine bubbles emerge continuously from the entire plumage, forming a smooth layer over the body and generating bubbly wakes behind the penguins. In several hours of film of hundreds of penguins, none were seen to swim rapidly upwards without bubbly wakes. Penguins descend and swim horizontally at about 2 meters/sec. Davenport et al. (2011) hypothesized that a significant proportion of the enhanced ascent speed is due to air lubrication reducing frictional and form drag and that buoyancy forces alone cannot explain the observed speeds.

Emperor Penguins create bubble trails (image: Blue Planet, BBC)
Links:
Why Divers Have Small Wings -- Many researchers
believe that small wings reduce drag underwater and, therefore, are
better
suited for diving. But until recently, there was no concrete evidence
for
the supposed benefits of small wings. Studying the effects of wing area
on diving is difficult; cross-species studies never give fair
comparisons.
Bridge (2004) decided to study the effect of altered wing size on
Common
Guillemots (Uria aalge) and Tufted Puffins (Fratercula
cirrhata)
during their brief molting periods.
Bridge (2004) used video cameras to film the
bird's diving
activity at SeaWorld California by mounting one camera in front of the
pool's But if reduced wing areas do not improve diving ability, why has natural selection favored small, pointed wings in many aquatic birds? Apparently birds with small, pointed wings are adept at high-speed, long-distance flight, essential for rapid movement between habitats. But, small, pointed wings cannot generate lift at low speed, so rapid vertical takeoffs are impossible. This is not a big problem for most diving birds because their open aquatic habitats prevent close approach by undetected predators. In addition, when the birds slow down to land, their small wings stall easily and lose lift. Fortunately, high-speed hard landings are more acceptable on water than on land. Thus, aquatic habitats relax the constraints on the evolution of small, pointed wings. -- Jane Qiu, Journal of Experimental Biology |
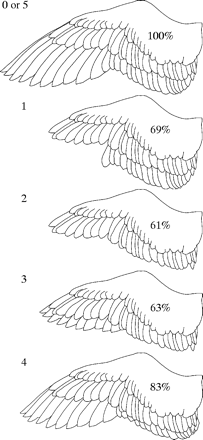
wing. Approximations of the percentage of intact wing area with the wing loosely extended are listed for each molt stage (Bridge 2004). |
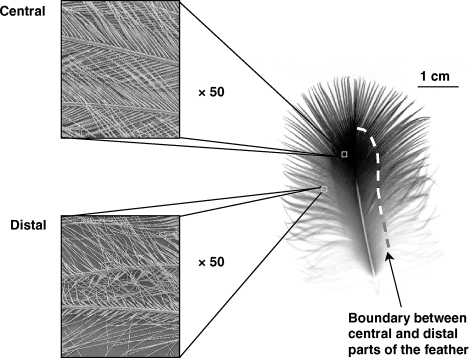

Great Cormorant feathers have a regular and highly waterproof central part whereas the distal region is irregular and wettable.
A ventral feather is shown (From: Grémillet et al. 2005).
Unusual feather structure of Great Cormorants -- Water has very high specific heat and thermal conductivity, so that diving endotherms can potentially
lose much heat to surrounding water.
To deal with this challenging environment, most warm-blooded divers have highly efficient body insulation.
Marine mammals have evolved thick
skins
and extensive peripheral fat layers, while diving birds have dense, highly waterproof plumage that traps an
insulating layer of air. A
few diving bird species, including Anhingas and cormorants are puzzling exceptions to this pattern, having plumage that is apparently
penetrated by water during submersion. The Great Cormorant (Phalacrocorax carbo) is thought to have a wettable plumage, providing low
body
insulation during foraging. Great Cormorants should thus be constrained by water temperatures, and show high energy requirements.
Surprisingly, this species has one of the widest breeding distributions of all diving birds, and does not require more food than these other species.
Grémillet et al. (2005) explored this apparent paradox by comparing the insulative properties of body plumage in four subspecies of great cormorants
ranging from tropical to polar regions. The authors found that all subspecies retained an insulating air layer in their plumage, which was, however, much
thinner than for other species of diving birds. Detailed examination of the plumage showed that each cormorant body feather has a loose,
instantaneously wet, outer section and a highly waterproof central portion. This indicates that the plumage of great cormorants is only partly wettable,
and that birds maintain a thin layer of air in their plumage. These findings suggest an unusual morphological-functional adaptation to diving which balances
the antagonist constraints of thermoregulation and buoyancy.
Double-crested Cormorant
Imperial Cormorant diving
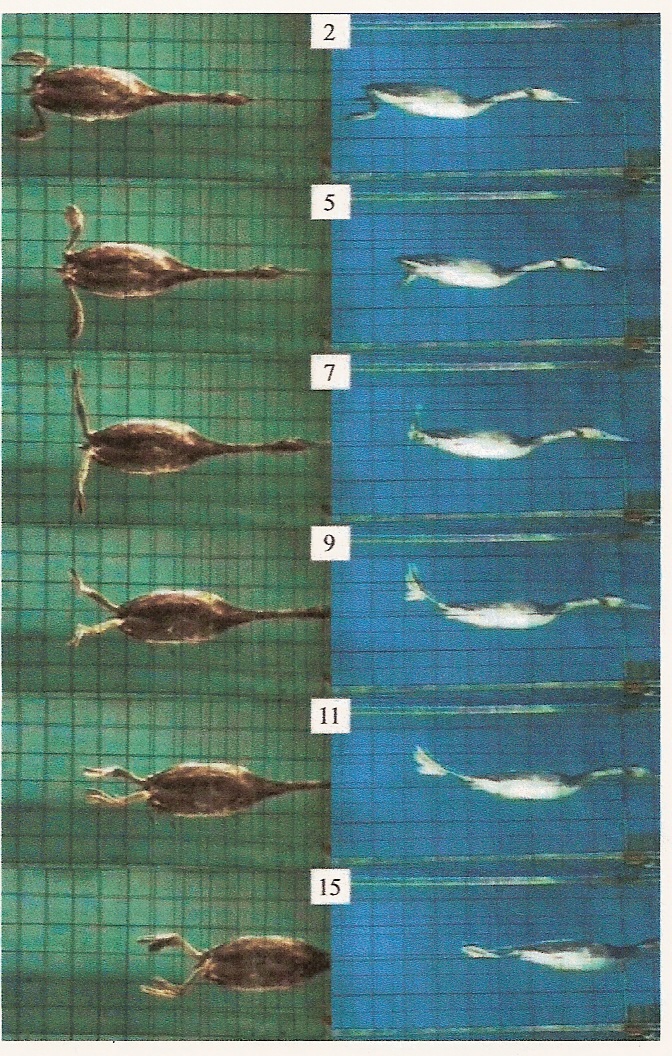 |
Foot-propelled locomotion -- When submerged, Great crested Grebes (Podiceps cristatus) swim with synchronized foot strokes, keeping their wings closely folded against the body. During the power stroke, the feet move from a cranial and ventrolateral position to a caudal and dorsomedial position relative to the body. The mean swimming speed varied from 0.7 - 1.2 meters/sec (Johansson and Norberg 2001). Dorsal (left) and lateral (right) video frames of a diving grebe. The dorsal view was recorded after reflection from a mirror. |
Western Grebe
Next: Bird Biogeography I
Lecture Notes:
Literature Cited:
Ákos, Z., M. Nagy, and T. Vicsek. 2008. Comparing bird and human soaring strategies. Proceedings of the National Academy of Sciences USA 105: 4139-4143.
Alexander, D. E. 2002. Nature's flyers: birds, insects, and the biomechanics of flight. Johns Hopkins University Press, Baltimore, MD.
Andersson, M. and J. Wallander. 2004. Kin selection and reciprocity in flight formation? Behavioral Ecology 15: 158-162.
Bejan, A. 2005. The constructal law of organization in nature: tree-shaped flows and body size. Journal of Experimental Biology 208: 1677- 1686.
Bridge, E. S. 2004. The effects of intense wing molt on diving in alcids and potential influences on the evolution of molt patterns. Journal of Experimental Biology 207: 3003 -3014.
Brighton, C. 2007. SYNOPSIS: Mechanical power output of Cockatiel flight in relation to flight speed. Biolog-e: The undergraduate bioscience research journal. University of Leeds. <http://www.fbs.leeds.ac.uk/students/ejournal/Biolog-e/uploads/Caroline%20Brighton_synopsis.pdf> (1 February 2008).
Cronin, T. W., M. R. Kinloch, and G. H. Olsen. 2005. Head-bobbing behavior in foraging Whooping Cranes favors visual fixation. Current Biology 15: R243-R244.
Davenport, J., R. N. Hughes, M. Shorten, and P. S. Larsen. 2011. Drag reduction by air release promotes fast ascent in jumping Emperor Penguins - a novel hypothesis. Marine Ecology Progress Series 430: 171-182.
Dial, K. P., A. A. Biewener, B. W.
Tobalske,
and D. R. Warrick. 1997. Mechanical power output of bird flight.
Science
390:67-70.
Grémillet, D., C. Chauvin, R. P. Wilson, Y, Le Maho, and S. Wanless. 2005. Unusual feather structure allows partial plumage wettability in diving Great Cormorants Phalacrocorax carbo. Journal of Avian Biology 36: 57-63.
Griffin, T.M. and R. Kram. 2000. Penguin waddling is not wasteful. Nature 408:929.
Hedrick, T. L., J. R. Usherwood and A. A. Biewener. 2004. Wing inertia and whole-body acceleration: an analysis of instantaneous aerodynamic force production in cockatiels (Nymphicus hollandicus) flying across a range of speeds. Journal of Experimental Biology 207: 1689-1702.
Johanssen, L. C. and U. M. L. Norberg. 2001. Lift-based paddling in diving grebe. Journal of Experimental Biology 204:1687-1696.
Kvist, A., A. Lindstrom, M. Green, T. Piersma, & G. H. Visser. 2001. Carrying large fuel loads during sustained bird flight is cheaper than expected. Nature 413: 730 - 732.
Louw, G. J. 1992. Functional anatomy of the penguin flipper. Journal of the South African Veterinary Association 63: 113-120.
Lovvorn, J. R. 2001. Upstroke thrust, drag effects, and stroke-glide cycles in wing-propelled swimming by birds. American Zoologist 41: 154-165.
Mangold, O. 1946. Die nase der segelnden Vo¨gel ein Organ des Stro¨mungssinnes? Naturwissenschaften 33:19-23.
Norberg, R. A. 1981. Why foraging birds in trees should climb and hop upwards rather than downwards. Ibis 123: 281-288.
Nyakatura, J. A., E. Andrada, N. Grimm, H. Weise, and M. S. Fischer. 2012. Kinematics and center of mass mechanics during terrestrial locomotion in Northern Lapwings (Vanellus vanellus, Charadriiformes). Journal of Experimental Zoology 9999A:1–15.
Ortega-Jimenez, V. M., and R. Dudley. 2012. Flying in the rain: hovering performance of Anna's Hummingbirds under varied precipitation. Proceedings of the Royal Society B 279: 3996-4002.
Osipova, E., et al. 2023. Loss of a gluconeogenic muscle enzyme contributed to adaptive metabolic traits in hummingbirds. Science 379: 185-190.
Pennycuick, C. J. 2002. Gust soaring as a basis for the flight of petrels and albatrosses (Procellariiformes). Avian Science 2: 1-12.
Pennycuick, C. J. 2008. Information systems for flying animals. In: Theoretical Ecology Series, vol. 5. Modelling the flying bird (C. J. Pennycuick, ed.), pp. 305-331. Elsevier Inc., Amsterdam, The Netherlands.
Sachs, G. 2005. Minimum shear wind strength required for dynamic soaring of albatrosses. Ibis 147: 1 - 10.
Song, J., H. Luo, and T. L. Hedrick. 2014. Three-dimensional flow and lift characteristics of a hovering Ruby-throated Hummingbird. Journal of the Royal Society Interface 11: 20140541.
Stowers, A. K., L. Y. Matloff, and D. Lentink. 2017. How pigeons couple three-dimensional elbow and wrist motion to morph their wings. Journal of the Royal Society Interface 14: 20170224.
Tobalske, B.W. and K.P. Dial. 1994. Neuromuscular control and kinematics of intermittent flight in Budgerigars (Melopsittacus undulatus). Journal of Experimental Biology 187:1-18.
Tobalske, B. W., N. E. Olson, and K. P. Dial. 1997. Flight style of the Black-billed Magpie: variation in wing kinematics, neuromuscular control, and muscle composition. Journal of Experimental Zoology 279: 313-329.
Tobalske, B. W., W. L. Peacock, and K. P. Dial. 1999. Kinematics of flap-bounding flight in the Zebra Finch over a wide range of speeds. Journal of Experimental Biology 202: 1725-1739.
Usherwood, J. R., M. Stavrou, J. C. Lowe, K. Roskilly, and A. M. Wilson. 2011. Flying in a flock comes at a cost in pigeons. Nature 474: 494-497.
Videler, J. J. 2005. Avian flight. Oxford University Press, Oxford, UK.
Warrick, D.R., B. W. Tobalske, and D. R. Powers. 2005. Aerodynamics of the hovering hummingbird. Nature 435: 1094-1097.
Weimerskirch, H., T. Guionnet, J. Martin, S. A. Shaffer, and D. P. Costa. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proceeding of the Royal Society B 267: 1869-1874.
Weimerskirch, H., J. Martin, Y. Clerquin, P. Alexandre, and S. Jiraskova. 2001. Energy saving in flight formation. Nature 413: 697 - 698.
Back to BIO 554/754 syllabus