|
Ornithology Introduction to Birds |
 |
 |
|
Ornithology Introduction to Birds |
 |
 |
Birds:
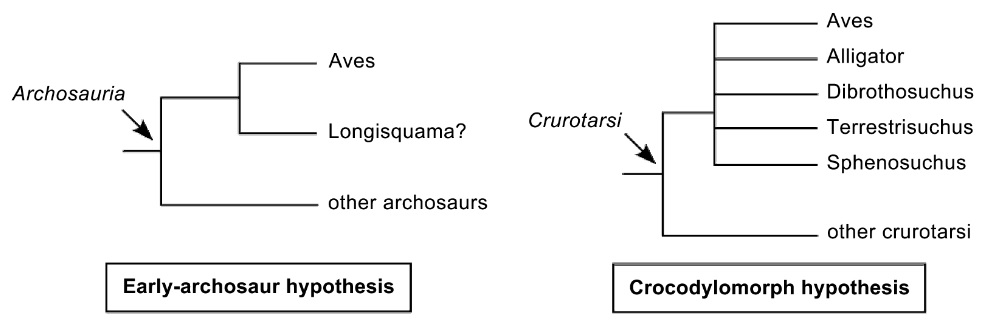
The Early-Archosaur Hypothesis posits that the origin of birds is more likely to be among early archosaurs (sometimes referred to as thecodonts) than among the theropod dinosaurs and that
similarities between theropods and birds are due to convergent evolution. The Crocodylomorph Hypothesis proposes that birds share a common ancestors with Crocodylomorpha or that the sister groups of birds
is within the Crocodylomorpha or that Aves is the sister clade of Crocodylia. The Crurotarsi (sometimes called Pseudosuchians) were a group of Archosaurs that flourished during the Triassic (251 - 200 million years ago).
These
hypotheses about the origin of birds are viewed as seriously flawed by most paleontologists because they are either based on a small number of
similarities from certain parts of the body between birds and the
proposed
groups or because
strongly biased data sets were used to generate supporting evidence (Xu et al. 2014) (Figure from James and Pourtless 2009).
For more information, check out The 'Birds Are Not Dinosaurs' Movement
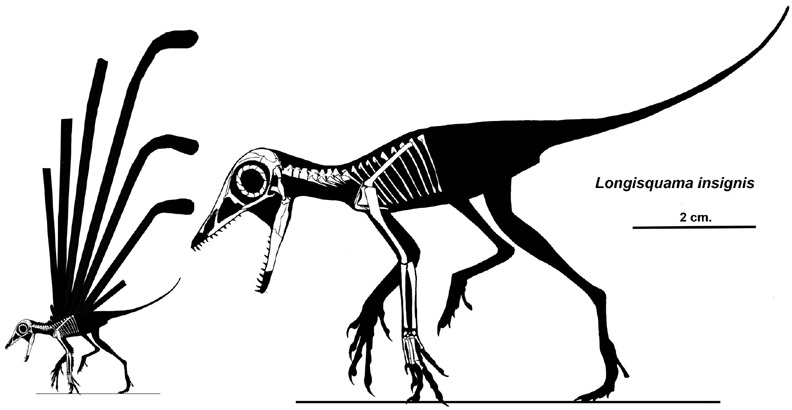
Source: https://en.wikipedia.org/wiki/Longisquama#/media/File:Longisquama_insignis_skeleton%26silhouette_small.jpg
Current consensus concerning bird evolution (Figure modifed from Brusatte et al. 2010).

A 75-million-year-old meat-eating dinosaur (Bambiraptor
feinbergi) has a number of features that look more bird-like
than dinosaur-like, providing evidence that birds may
have evolved from dinosaurs.
Source: http://exn.ca/dinosaurs/home.cfm?id=20000321-56&SubType=BirdDino
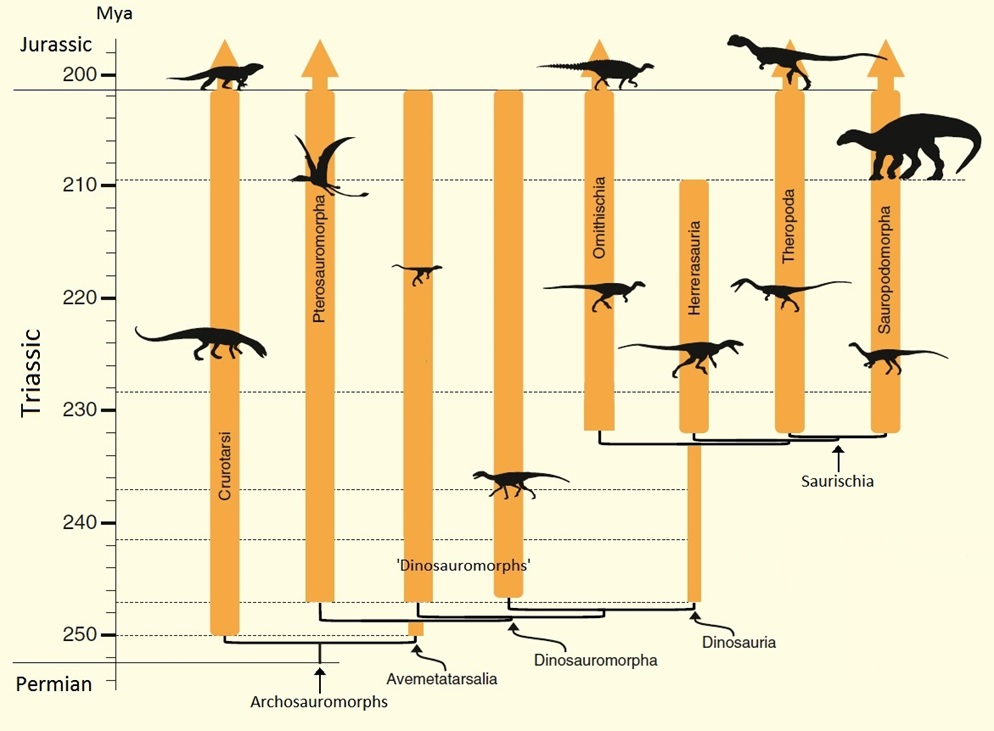
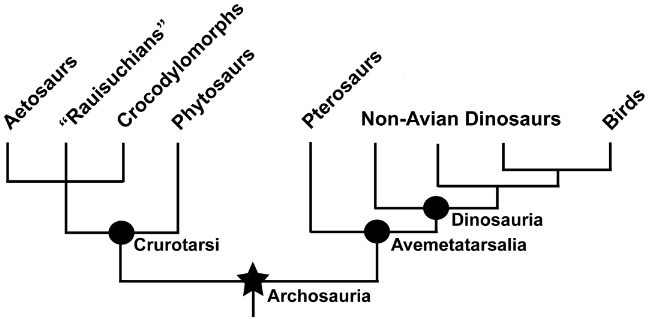
Major groups of archosaurs, including crurotarsi, or the crocodile line, and the avemetatarsalia, or the bird line.
Only the crurotarsi, pterosauromorphs, and dinosaurs survived into the Jurassic. The Herrerasauria were basal theropods that did
not survive into the Jurassic. During the Triassic, Dinosauria diverged into three major groups, one that did not survive into the
Jurassic (Herrerasaura, or basal Theropods), and two that did, Ornithischia and Saurischia. The Saurichians then diverged into Theropoda
and Sauropodomorpha (Figure modified a bit from Benton et al. 2014).
Video: The origin of birds
Theropod anatomy
Feathered dinosaur
Dr. Jingmai O'Connor - The evolution of dinosaurian flight & the rise of birds
Origin of avian genome size and structure in non-avian dinosaurs -- Avian genomes are small and streamlined compared with those of other amniotes, with fewer repetitive elements and less non-coding DNA (a typical bird genome consists of about 1.45 billion base pairs; human genomes are another billion base pairs longer). This condition has been suggested to represent a key adaptation for flight in birds, by reducing the metabolic costs associated with having large genome and cell sizes. However, the evolution of genome architecture in birds, or any other lineage, is difficult to study because genomic information is often absent for long-extinct relatives. Organ et al. (2007) found that bone-cell size correlates well with genome size in extant vertebrates, and used that relationship to estimate the genome sizes of 31 species of extinct dinosaur, including several species of extinct birds. Their results indicate that the small genomes typically associated with avian flight evolved in the saurischian dinosaur lineage between 230 and 250 million years ago, long before this lineage gave rise to the first birds. By comparison, ornithischian dinosaurs were inferred to have had much larger genomes, probably typical of ancestral Dinosauria. Using comparative genomic data, Organ et al. (2007) estimated that genome-wide interspersed mobile elements, a class of repetitive DNA, comprised 5–12% of the total genome size in the saurischian dinosaur lineage, but was 7–19% of total genome size in ornithischian dinosaurs, suggesting that repetitive elements became less active in the saurischian lineage. These genomic characteristics should be added to the list of attributes previously considered avian, but now thought to have arisen in non-avian dinosaurs, such as feathers, pulmonary innovations, and parental care and nesting.
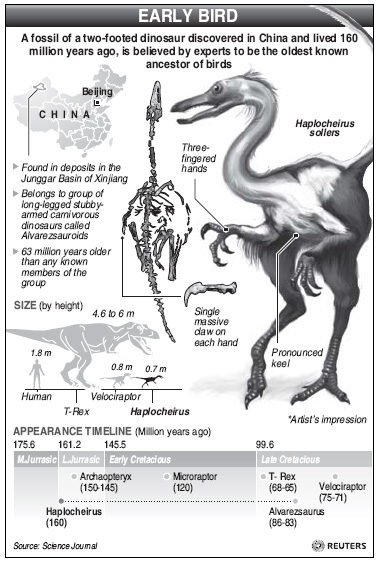 |
 Haplocheirus sollers |
Bird-dinosaur link strengthened -- The fossil record of Jurassic theropod dinosaurs closely related to birds remains poor. Choiniere et al. (2010) reported a new theropod, Haplocheirus sollers (meaning simple, skillful hand), from the earliest Late Jurassic of western China represents the earliest diverging member of the enigmatic theropod group Alvarezsauroidea and confirms that this group is a basal member of Maniraptora, the clade containing birds and their closest theropod relatives. It extends the fossil record of Alvarezsauroidea by 63 million years and provides evidence for maniraptorans earlier in the fossil record than Archaeopteryx. The new taxon confirms extreme morphological convergence between birds and derived alvarezsauroids and illuminates incipient stages of the highly modified alvarezsaurid forelimb.
Jonah Choiniere Describes A Newly Discovered Dinosaur Haplocheirus sollers
Related links:
Dinosaur discovery helps solve piece of evolutionary puzzle
New dinosaur discovery solves evolutionary bird puzzle

Sinosauropteryx
Fossilized melanosomes and the color of Cretaceous dinosaurs and birds -- Spectacular fossils from the Early Cretaceous Jehol Group of northeastern China have greatly expanded our knowledge of the diversity and palaeobiology of dinosaurs and early birds, and contributed to our understanding of the origin of birds, of flight, and of feathers. Pennaceous (vaned) feathers and integumentary filaments are preserved in birds and non-avian theropod dinosaurs, but little is known of their microstructure. Zhang et al. (2010) report that melanosomes (color-bearing organelles) are not only preserved in the pennaceous feathers of early birds, but also in an identical manner in integumentary filaments of non-avian dinosaurs, thus refuting recent claims that the filaments are partially decayed dermal collagen fibers. Examples of both eumelanosomes and phaeomelanosomes have been identified, and they are often preserved in life position within the structure of partially degraded feathers and filaments. Furthermore, these data provide empirical evidence for reconstructing the colors and color patterning of these extinct birds and theropod dinosaurs. For example, the dark-colored stripes on the tail of the theropod dinosaur Sinosauropteryx can reasonably be inferred to have exhibited chestnut to reddish-brown tones.
Related links:Study offers an insight into dinosaur colors
Microraptor's Feather Color Revealed
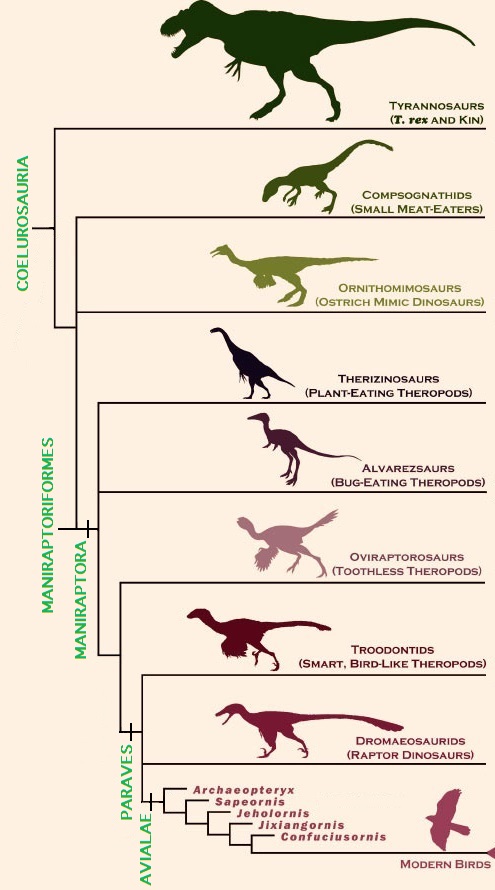
Interrelationships of the major taxa of the Coelurosauria (Figure from Brusette et al. 2014).
Video: how did T-rex walk?
Video: Compsognathus
Video: Oviraptorid (Oviraptorosaurid) fights to protect nest Video: Microraptor
(Dromaeosauridae) - Flying dinosaur Selected species illustrating archosauromorphan phylogeny and the evolution of characteristics through the appearance of the Coelurosauria. Appearance of key traits in the evolution of birds. Among birds (Aves), available fossils provide evidence for the presence of a crop and loss of the right ovary in Jeholornis (O’Connor and Zhou 2015), Jurassic maniraptoran with elongate ribbon-like feathers -- Recent coelurosaurian discoveries have greatly enriched our knowledge of the transition from dinosaurs to birds, but all reported taxa close to this transition are from relatively well known coelurosaurian groups. Zhang et al. (2008) reported a new basal avialan, Epidexipteryx hui, from the Middle to Late Jurassic of Inner Mongolia, China. This new species is characterized by an unexpected combination of characters seen in several different theropod groups, particularly the Oviraptorosauria. Phylogenetic analysis shows it to be the sister taxon to Epidendrosaurus, forming a new clade at the base of Avialae. Epidexipteryx also possessed two pairs of elongate ribbon-like tail feathers, and its limbs lack contour feathers for flight. Epidexipteryx's ribbon-like tail feathers could have served as ornamentation as well as balancing tools for help with moving along tree branches. Shorter feathers also covered the dinosaur's body and could have served as insulation. This finding shows that a member of the avialan lineage experimented with integumentary ornamentation as early as the Middle to Late Jurassic, and provides further evidence relating to this aspect of the transition from non-avian theropods to birds.
Video: Troodontid from Mongolia
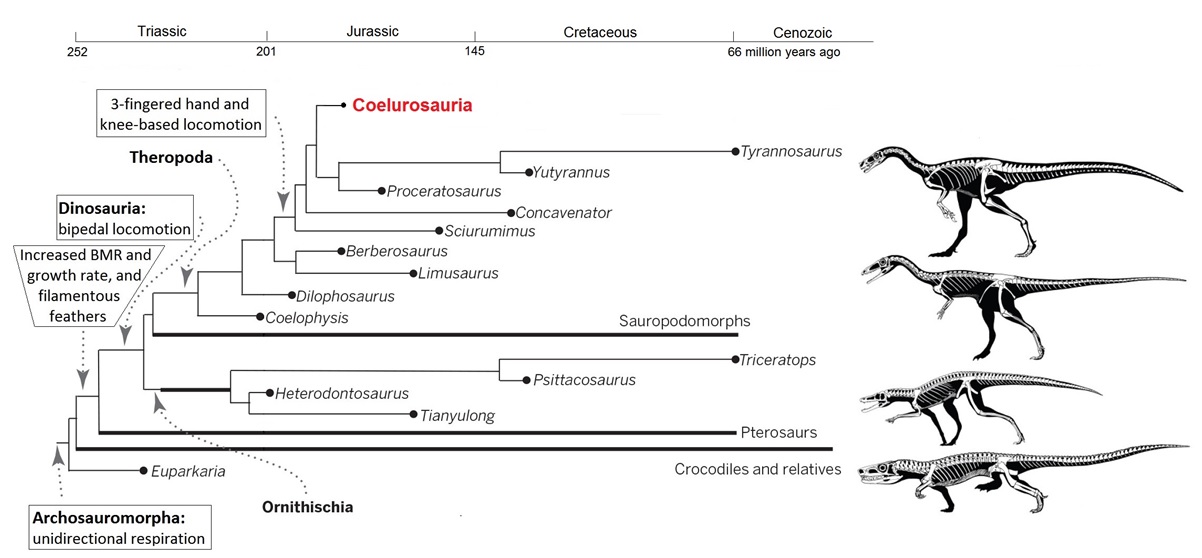
Tyrannosaurs represent the basal coelurosaurs and they already had several bird-like characteristics, including unidirectional respiration, bipedal and increasingly
knee-based locomotion,
three-fingered hands, filamentous feathers, faster growth rates, and higher basal metabolic rates (Figure modified from Xu et al. 2014). 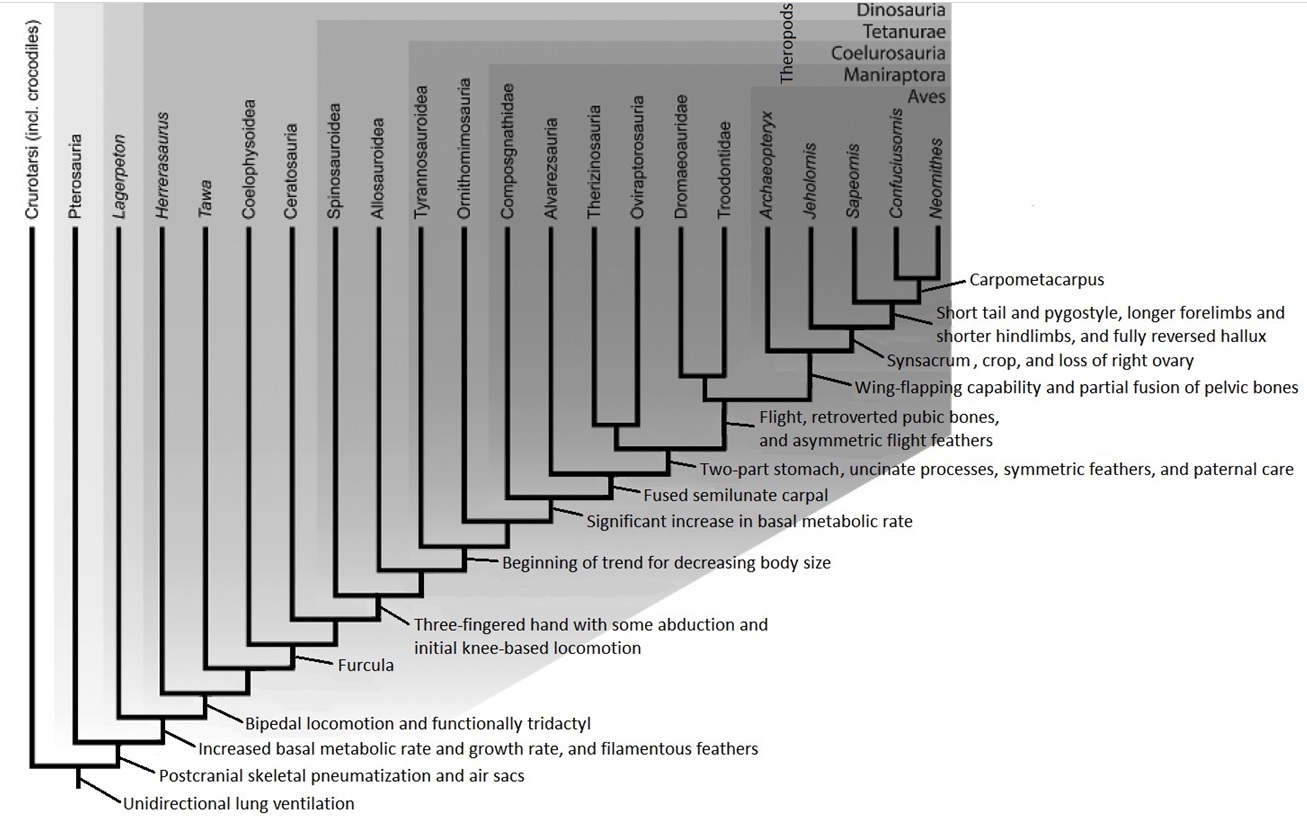
but such evidence is not available for Archaeopteryx. Information provided in this figure obtained from Butler et al. (2009), Makovicky and Zanno (2011), Heers and Dial (2011), Rashid et al. (2014),
Han et al. (2014),
Ksepka (2014), Li et al. (2014), O’Connor and Zhou (2015) (Figure greatly modified from Makovicky and Zanno 2011).

Epidexipteryx hui. a, Main slab; b, c, skull in main slab (b) and counterslab (c); d, four elongate ribbon-like tail feathers; b', c', line drawings of b and c, respectively. Abbreviations: l1, l2 and l7, 1st, 2nd and 7th left teeth of upper jaw; l1', r1' and r5', 1st left, 1st right and 5th right teeth of lower jaw; l2 and r2, 2nd left and right teeth of upper jaw.

Epidexipteryx hui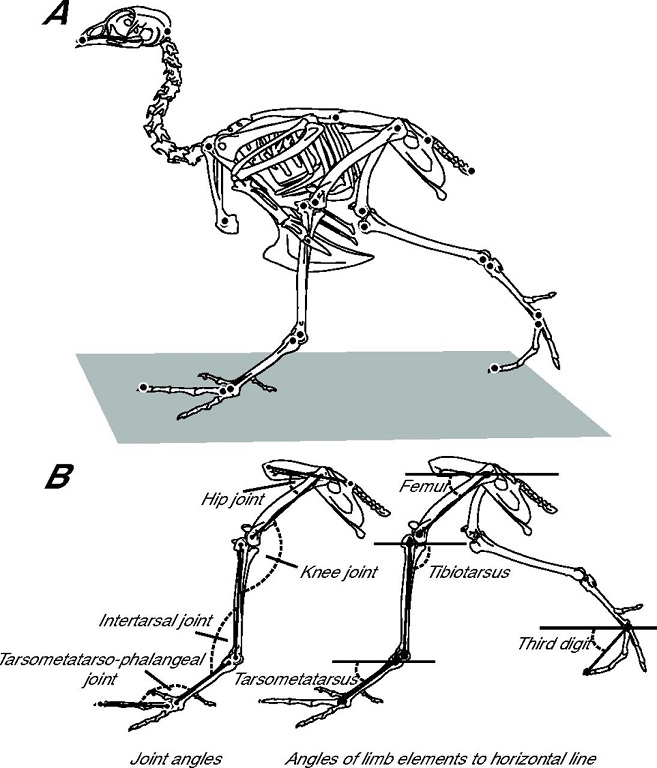
Knee-based locomotion. In contrast to bipedal humans where, when walking or running, most of the movement is at
the hip jount,
most of the movement in the bird leg is at the knee joint (Figure from Stoessel and Fischer 2012).
"Archosauria is the clade composed of the most recent common ancestor of birds and their closest living relatives, crocodilians, as well as all of its descendants (see Figure above). On the basis of shared derived morphological characters of the ankle, birds are placed in one of two major lineages of archosaurs, the one that includes both pterosaurs and dinosaurs. Within Dinosauria, birds as a clade are strongly supported by skeletal characters as one lineage of a clade that includes a variety of small raptor dinosaurs. Birds are placed as part of Avialae in the clade Maniraptora, which is part of the progressively more inclusive dinosaurian clades Theropoda and Saurischia. The evolution of both terrestrial and aerial locomotion in the Dinosauria as well as temporal patterns of dinosaur diversification and extinction are the subjects of active research." - Clark and Middleton (2006).
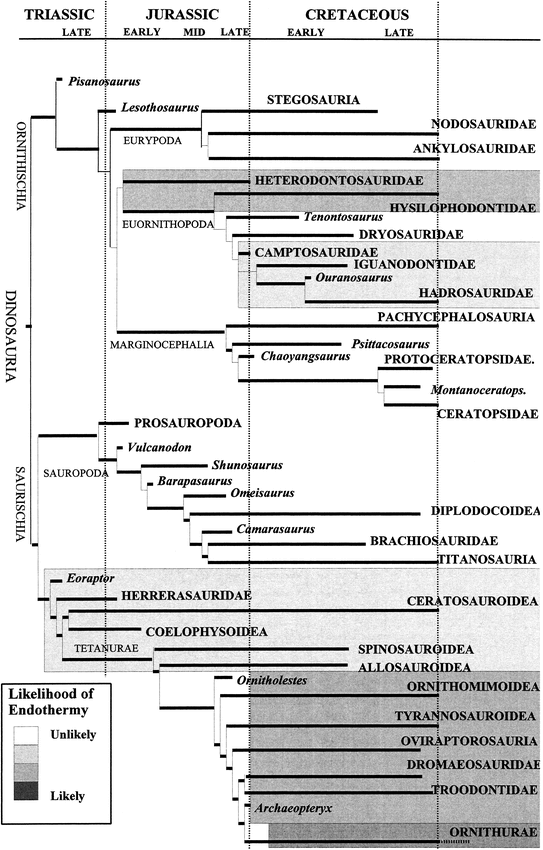
Evolution of endothermy -- Present-day birds are endothermic and, of course, the primitive state among vertebrates is ectothermy. Seebacher (2003) presented a speculated phylogenetic distribution of endothermy among the Dinosauria. Endothermy must have evolved sometime in the lineage leading to modern birds (Ornithurae, very dark shading) and is likely to have occurred in coelurosaurs that exhibited an evolutionary trend toward a decrease in body size, and also lived at mid- to high latitudes (dark shading). It is less likely that endothermy evolved among other theropods that showed an evolutionary trend toward large body size (light gray shading), or among any other group of dinosaurs in which the most recent members attained large body size (very light gray shading). Hypsilophodontids and heterodontosaurids remained small and occurred at mid- to high latitudes, so endothermy may have been of selective advantage in those dinosaurs (gray shading).
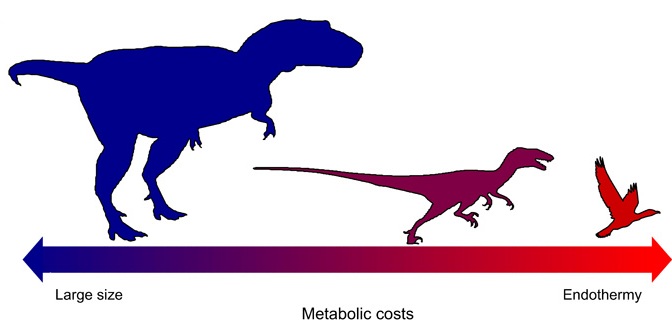
A reduction in body size from ancestral theropods to basal birds likely represents the evolutionary path of least resistance
for the evolution of avian endothermy, with the energy costs of being large traded for those of being endothermic (From: Rezende et all 2020).
 With jagged teeth and raptor-like features, the feathered Archaeopteryx is unlike any modern species of bird (Image: G. Mayr/Senckenberg) |
|
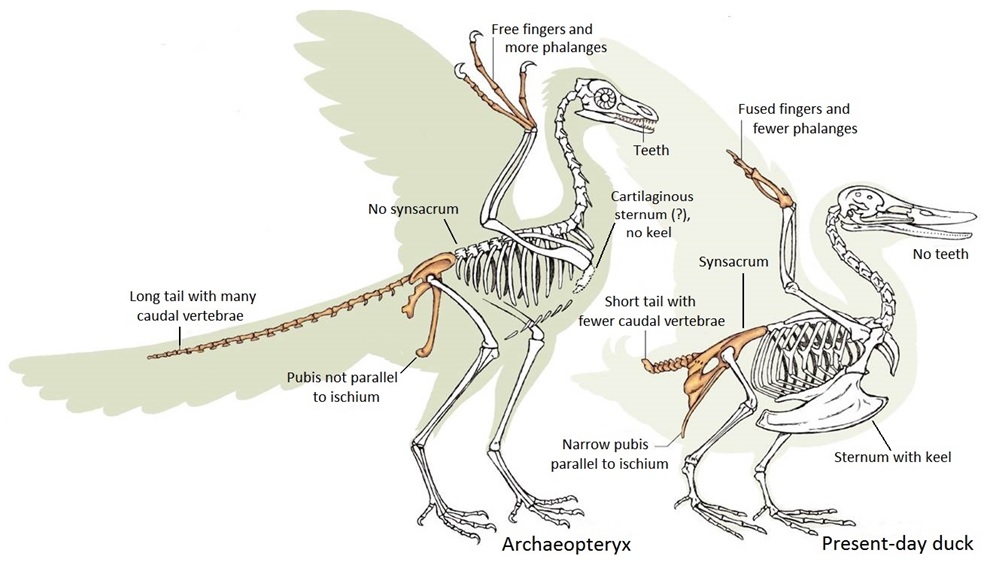
A well-preserved Archaeopteryx specimen with theropod features -- A nearly complete skeleton of Archaeopteryx with excellent bone preservation shows that the osteology is similar to that of non-avian theropod dinosaurs. This new specimen confirms the presence of a hyperextendible second toe as in dromaeosaurs and troodontids. Archaeopteryx had a plesiomorphic tetraradiate palatine bone (shaped in the same way as in many two-legged dinosaurs) and no fully reversed first toe (or hallux). These observations provide further evidence for the theropod ancestry of birds. In addition, the presence of a hyperextendible second toe blurs the distinction of archaeopterygids from basal deinonychosaurs (troodontids and dromaeosaurs) and challenges the monophyly of Aves (From Mayr et al. 2005). Deinonychosaurs included the famous Velociraptor. Generally, deinonychosaurs were small and lightly built, with deadly teeth and a distinctive sickle-shaped claw on their second toe, which was perfect for disembowelling prey.
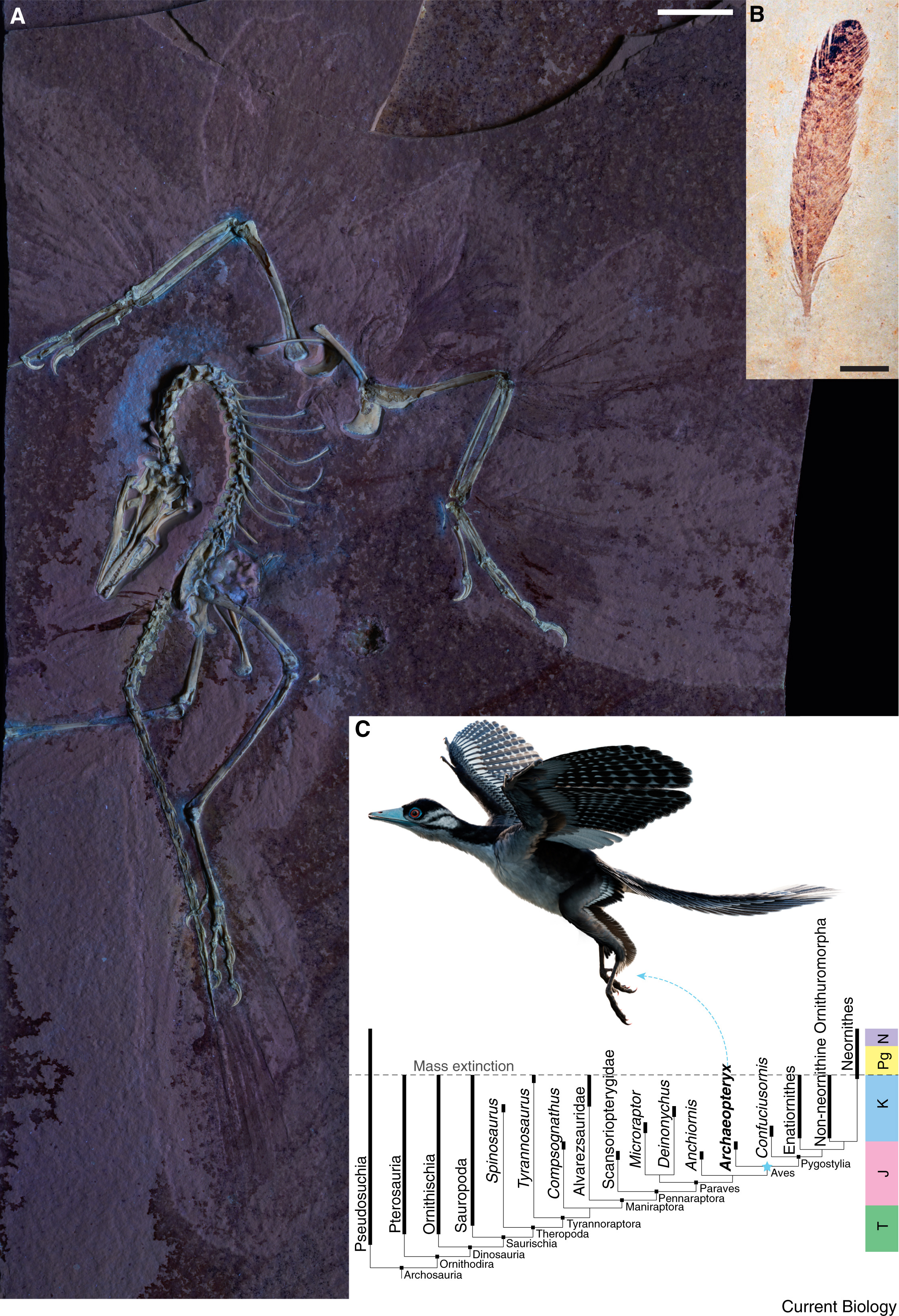
(A) The Chicago Archaeopteryx FMNH PA 830 (the 14th skeletal specimen). (B) The first described Archaeopteryx specimen,
an isolated feather (image: Luis Chiappe). (C) Simplified phylogeny of archosaur reptiles depicting
the placement of Archaeopteryx within Aves and the Dinosauria more broadly (From: O'Connor 2025).
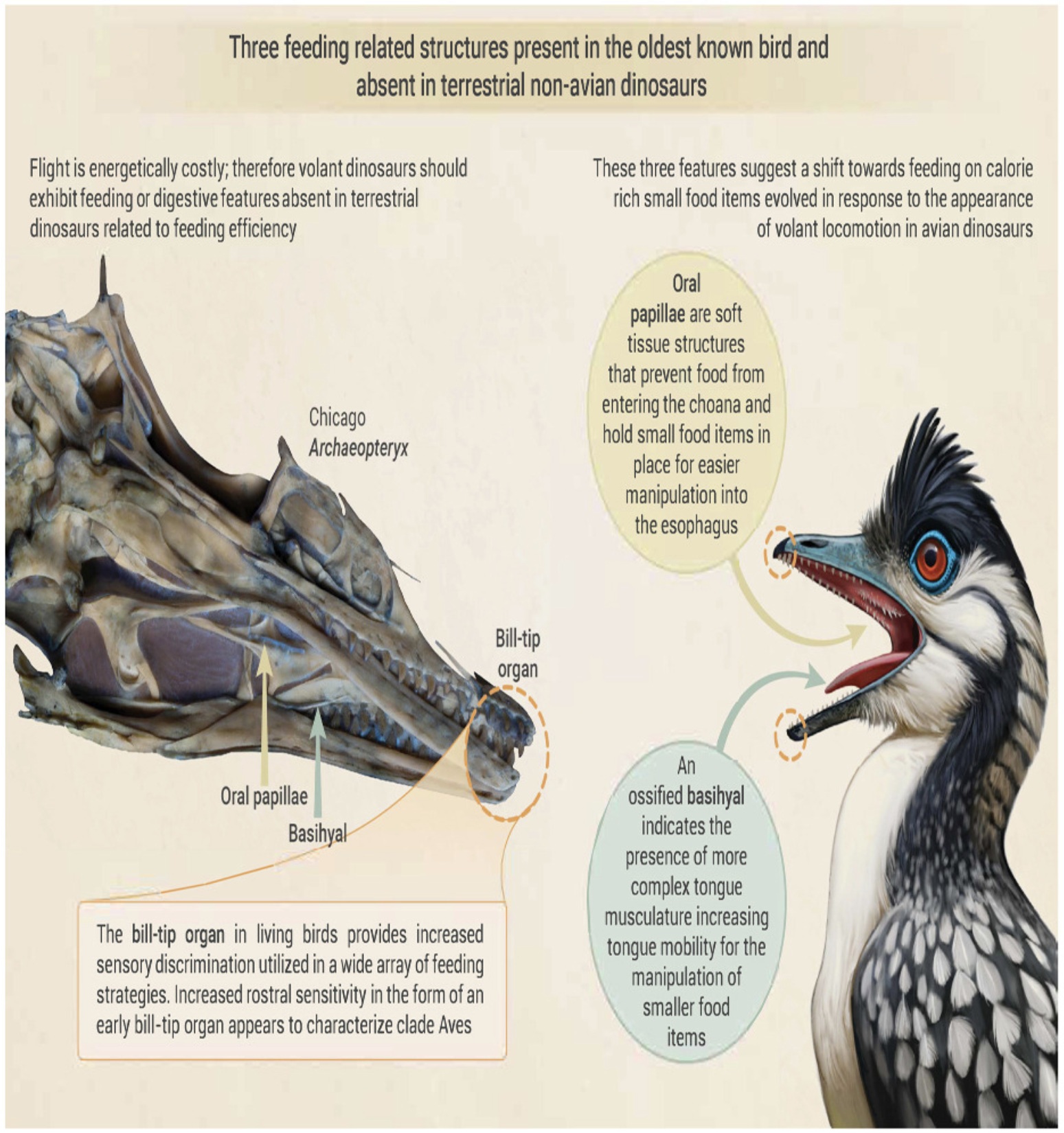
Powered flight, as the most physically demanding form of vertebrate locomotion, involves elevated energetic demands.
The appearance of dinosaurian flight should therefore be associated with novel features related to increased feeding or
digestive efficiency. Neornithines have several unique rostral features that facilitate complex oral tasks, increasing feeding
efficiency; these include a mechanoreceptive bill-tip organ, highly mobile tongue, and oral papillae. O'Connor et al. (2026)
provide the first evidence for the presence of these features in Archaeopteryx, the oldest known avian dinosaur. Large
neurovascular openings at the tip of both the upper and lower jaw indicate the neurovasculature contained within the
sensitive rostrum of non-avian theropods exited the tip of the snout. This suggests the presence of a mechanoreceptive
soft tissue structure that was apparently widespread in early-diverging toothed birds, adding to the recognized structural
diversity of the avian bill-tip organ. The co-occurrence of soft tissue oral papillae and an ossified basihyal indicative of
increased tongue mobility is consistent with the interdependence of these structures in extant birds. The appearance of
these three features close to the origin of feather-driven flight suggests that they evolved through pressures imposed by
the increased caloric demands associated with the transition from terrestrial predator to volant bird.

Reconstruction of the plumage of Archaeopteryx by Michael Rothman. The black and white pattern of the feathers
is based on previous
research that indicates the isolated wing covert was black and white (Carney et al., 2012). This
Chicago specimen reveals the presence of the enlarged tertial tract (From: O'Connor et al. 2025).
Archaeopteryx: the world's most famous bird
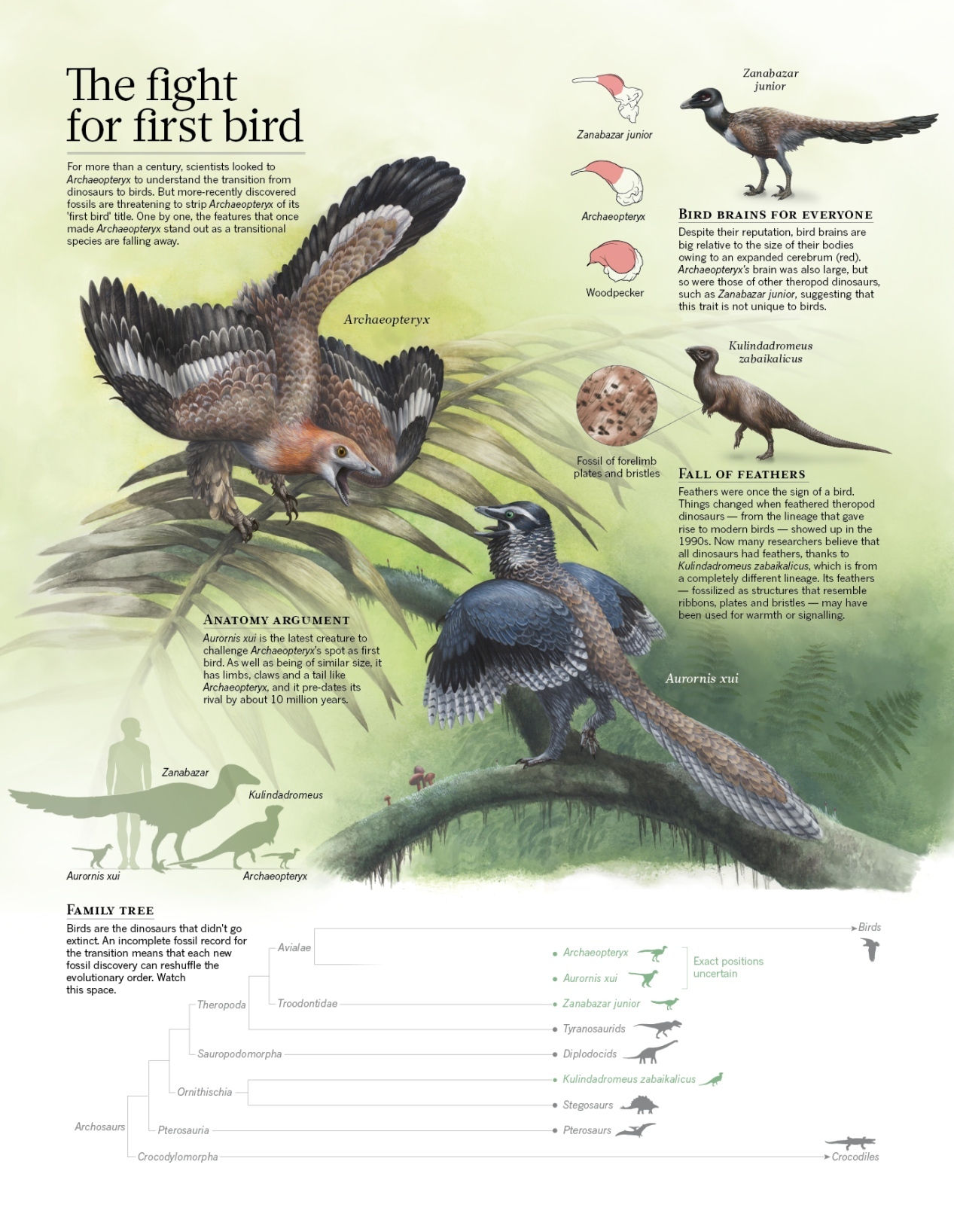
From: Callaway (2014)
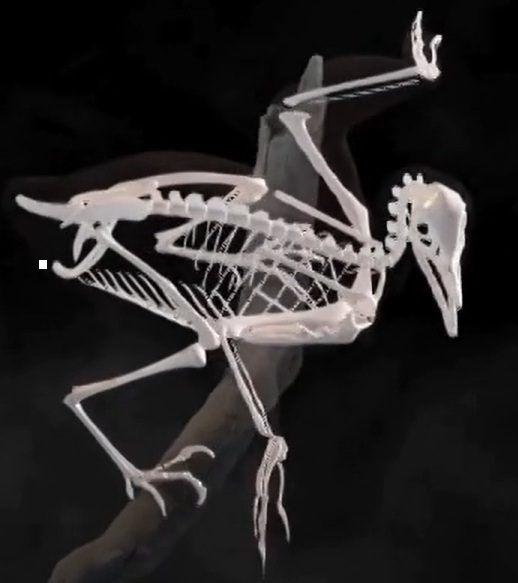
Baminornis zhenghensis
Fossils of Archaeopteryx are some of the most famous in history, but it is also an enigma. For more than a century, Archaeopteryx has been the only known bird genus from the Jurassic: the period when birds first evolved. In 2025, a second genus of Jurassic birds, Baminornis, was discovered and described in China. Baminornis is unlike Archaeopteryx, hinting at a complex evolutionary story. Baminornis was a small bird with a mass of 140–300 grams. Most strikingly, the five lowest vertebrae of the tail had fused together to form a pygostyle. This is the norm in modern birds and is also seen in Cretaceous birds, but is absent in Archaeopteryx. A Jurassic bird with a fully formed pygostyle suggests that the first birds might have predated Archaeopteryx and Baminornis. This new fossil indicates the early appearance of highly derived bird features, and suggests an earlier origin of birds and a radiation of early birds in the Jurassic (From: Marshall 2026).

Baminornis zhenghensis
China Jurassic Fossil Discovery Sheds Light on Bird Origin
Earliest short-tailed bird from the Late Jurassic of China
Baminornis more closely resembles modern birds than Archaeopteryx, in two ways. First, it lacks the long tail seen in raptor dinosaurs and in Archaeopteryx; instead, some of its vertebrae are fused into a pygostyle. Second, the shoulder girdle of Baminornis is refashioned so that the scapula and coracoid bones are separate and the coracoid is strutshaped-adaptations associated with the musculature and arm motions of flapping flight. These anatomical features indicate that Baminornis was probably better at flying than Archaeopteryx, and maybe even have been a better flyer than other primitive birds from several million years later in the Cretaceous period. However, Baminornis did not have a large breastbone and keel so it’s unclear how big its flight muscles were and where they attached. ALso, its hand looks similar to that of a raptor dinosaur: individual fingers that could grab and slash, each with many bones, rather than the solid fused structure that secures the primary wing feathers of birds today. And, because its feathers have not been preserved, we don’t know how the wings of Baminornis were constructed, or even how big they were. Even the presence of a pygostyle doesn’t prove that Baminornis had strong flight capabilities because many dinosaurs closely related to birds also evolved shortened tails for various reasons. Ultimately, to understand how Baminornis flew, rigorous biomechanical modelling and hypothesis testing will be needed, and this will be a fruitful next step in research on this remarkable fossil. No matter how acrobatic Baminornis was in the air, this fossil provides evidence that birds were more diverse early in their history than was previously thought. There was probably a bevy of birds-long-tailed ones similar to Archaeopteryx, short-tailed ones such as Baminornis, maybe even muscular flappers, flying over the heads of Brontosaurus, Stegosaurus and other Jurassic dinosaurs. More fossils must be out there, in those rare places that can preserve delicate skeletons, so let’s keep looking (From: Brusatte 2025).
Bird evolution: a summary -- The study of bird origins is over 150 years old. The dinosaurian origin of birds gained broad support after the resemblance between birds and theropods was first recognized by Huxley (1868) and other paleontologists. In Heilmann's (1926) classic book ("The Origin of Birds"), he suggested that, despite the similarity between birds and theropods, dinosaurs were probably too specialized to be the direct ancestors of birds and proposed that birds and dinosaurs probably evolved from a common ancestor in a group called Thecodontia. Heilmann's proposal was so authoritative and influential that the thecodont origin of birds became the prevalent hypothesis for nearly half a century. The resurrection of the dinosaurian–bird hypothesis by John Ostrom in the 1970s (Ostrom 1976), with the support of cladistic analysis since the 1980s, has resulted in a general consensus among many paleontologists about the validity of the dinosaurian–bird hypothesis. The discovery of many new and better preserved theropods in the past two decades, particularly those with feather impressions from the Lower Cretaceous of Liaoning, have provided some of the most compelling evidence supporting the hypothesis (Zhou 2004). In more recent years, several additional findings provide yet more support for the dinosaur-bird hypothesis, including molecular evidence of the link between birds and dinosaurs (noted above), the small genomes of birds and saurischian dinosaurs (noted above), similarities in the respiratory systems of birds and theropods, the presence of uncinate processes in both birds and theropods (see below for more details), and similarities between birds and certain theropods in aspects of parental care and nesting. As a result, the generally accepted consensus is that birds are dinosaurs.
| The avian nature of the brain and inner ear of Archaeopteryx (Alonso et al. 2004) - Archaeopteryx, the earliest known flying bird from the Late Jurassic period, exhibits many shared primitive characters with more basal coelurosaurian dinosaurs (the clade including all theropods more bird-like than Allosaurus), such as teeth, a long bony tail and pinnate feathers. However, Archaeopteryx possessed asymmetrical flight feathers on its wings and tail, together with a wing feather arrangement shared with modern birds. This suggests some degree of powered flight capability but, until now, little was understood about the extent to which its brain and special senses were adapted for flight. Alonso et al. (2004) investigated this problem by computed tomography scanning and three-dimensional reconstruction of the braincase of the London specimen of Archaeopteryx. A reconstruction of the braincase and endocasts of the brain and inner ear suggest that Archaeopteryx closely resembled modern birds in the dominance of the sense of vision and in the possession of expanded auditory and spatial sensory perception in the ear. Alonso et al. (2004) concluded that Archaeopteryx had acquired the derived neurological and structural adaptations necessary for flight. An enlarged forebrain suggests that it had also developed enhanced somatosensory integration with these special senses demanded by a lifestyle involving flying ability (For more detail check: The early origin of a birdlike inner ear and the evolution of dinosaurian movement and vocalization). | 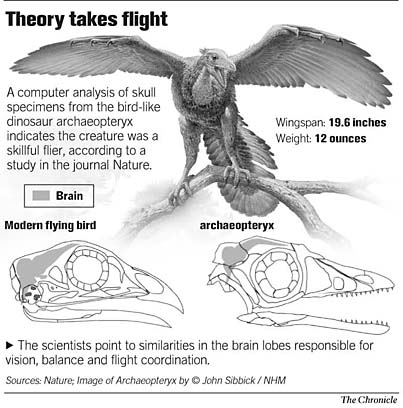
|
Color of an Archaeopteryx feather -- Archaeopteryx has been regarded as an icon of evolution ever since its discovery from the Late Jurassic limestone deposits of Solnhofen, Germany in 1861. Carney et al. (2012) report the first evidence of color from Archaeopteryx based on fossilized colour-imparting melanosomes discovered in this isolated feather specimen. Using a phylogenetically diverse database of extant bird feathers, statistical analysis of melanosome morphology predicts that the original colour of this Archaeopteryx feather was black, with 95% probability. Furthermore, reexamination of the feather's morphology indicates it was an upper major primary covert, contrary to previous interpretations. Additional findings reveal that the specimen is preserved as an organosulphur residue, and that barbule microstructure identical to that of modern bird feathers had evolved as early as the Jurassic. As in extant birds, the extensive melanization would have provided structural advantages to the Archaeopteryx wing feather during this early evolutionary stage of flight.
Link:
Flying dinosaur had black feathers
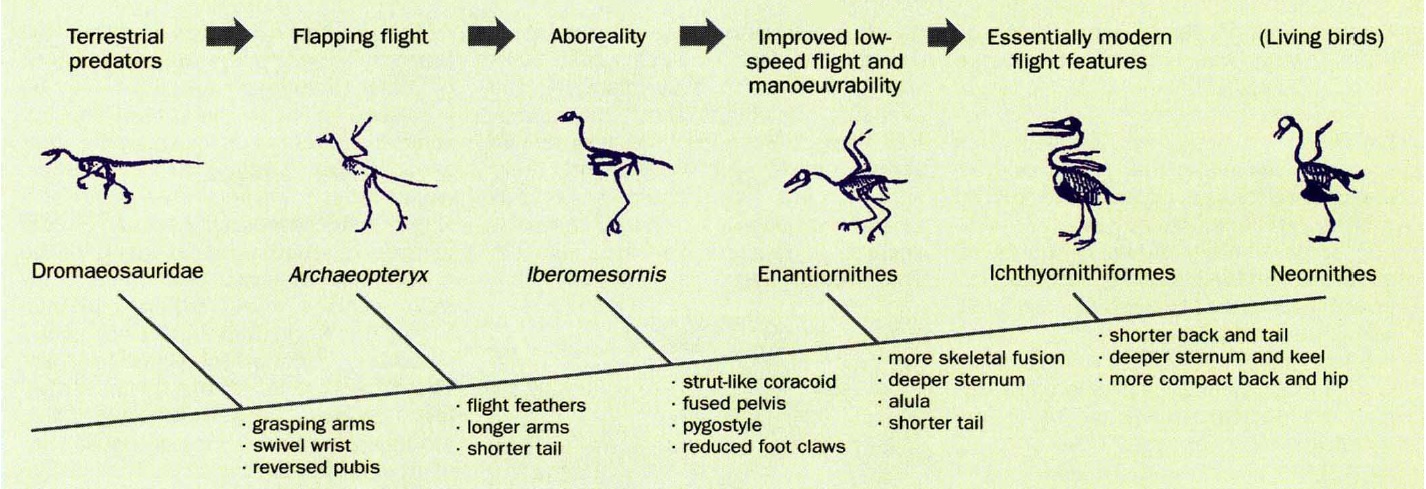
From Padian (1996)
Inferring the Phylogenetic Origin of Avian Powered Flight
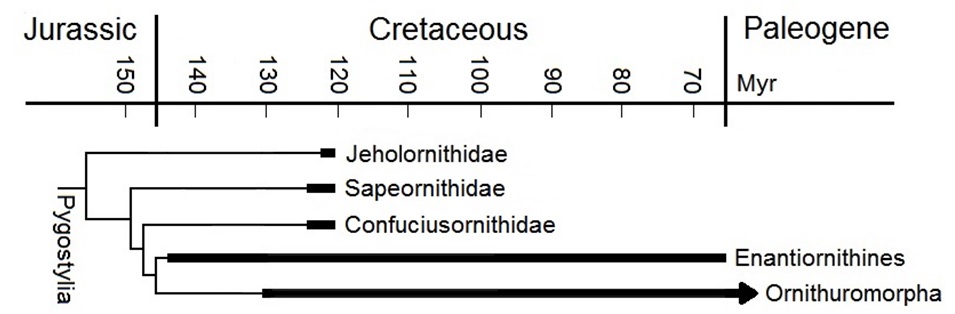
Major groups of Mesozoic birds included the Jeholornithidae, Saperornithidae, Confuciusornithidae, Enantiornithines, and Ornithuromorpha.
Enantiornithines were the dominant birds during the Cretaceous.
The oldest ancestors of present-day birds, the Ornithuromorphs, first appear in the fossil record
about 131 million years ago.
Note that over a period of about 2 – 3 million years just prior to 120 million years ago, all five groups of pygostylian birds coexisted.
The Enantiornithes are an extinct lineage of stem-bird, meaning they are members of the group Aves (or Avialae), but with no living descendants. This extinct group of birds was mostly arboreal and dominated terrestrial environments from 130 to 66 million years ago. With approximately 90 known genera, they account for more than half of the known diversity of Mesozoic birds. Yet, despite apparently out-competing birds more closely related to living species in most Cretaceous continental environments, enantiornithines mysteriously went extinct at the end of the Cretaceous.
Available information indicates that enantiornithines were highly precocial, hatching with a well ossified skeleton and fully developed remiges, presumably fully independent and capable of flight within the first 24 hours after hatching. Bone microstructure indicates that this locomotor independence involved the usual energetic trade-off because enantiornithines grew slowly and for several years, whereas almost all living birds are born incapable of flight and grow rapidly, reaching adult size in a matter of weeks.
As in present-day birds, the tail is the most variable part of enantiornithine plumage. Most tail shapes are thought to be ornamental and the absence of rectrices in most specimens is inferred to be indicative of sexual dimorphism, with the specimens possessing ornamental tail feathers being males and those without rectrices being females.

Cretaceous enantiornithines. Top: male Feitianius; center left: female
Feitianius; center: Orienantius;
center right: Sulcavis . Bottom left: Avimaia; bottom center: Falcatakely; bottom right: Longipteryx. Scale bar = 5 cm.
Modern birds use a variety of flight styles from continuous flapping flight to soaring, which involves very little flapping. Aerodynamic analysis suggests that Early Cretaceous enantiornithines used intermittent flapping flight styles like bounding (bursts of flapping punctuated by short intervals where the wings are folded against the body) and flap-gliding. However, these flight styles are limited to birds with body masses less than 300 grams. Larger enantiornithines that evolved in the Late Cretaceous must have used different flight styles, although the fragmentary nature of specimens from younger deposits defies precise analysis. As far as diet, enantiornithines were polyphylodont, meaning their teeth were continuously replaced throughout their lifetime. Tooth shapes vary enormously among enantiornithines, hinting at a diversity of diets and suggestive of resource partitioning.
Morphology suggests that by the Late Cretaceous enantiornithines had refined their flight apparatus, increased skeletal pneumaticity (invasion of the skeleton by air sacs), evolved beaks and increased growth rates — features that all are associated with present-day birds. So why did this very lineage go extinct? It is unlikely that a single factor can explain the extinction of enantiornithines. Most likely a combination of physiological differences allowed neornithines to survive when enantiornithines did not. Although elevated growth rates evolved in at least some Late Cretaceous enantiornithine lineages, development was still slow compared to crownward birds. The half-buried morphology of their nests may also have been an important factor (i.e., eggs were
partially embedded in sediment), limiting their ability to recover clutches and indicating relatively longer incubation periods. Together with major losses in habitat due to forest fires, these physiological limitations may have led to the demise of this successful avian lineage (From: O'Connor 2022).

Drawing of an Enantiornithine bird, Cratoavis cearensis, from the Early Cretaceous of Brazil. The
rectrices were about 7 cm long and, from bill tip to the end of the tail feathers, Cratoavis was only about
12 cm long (From Carvalho et al. 2015; drawing by Deverson Pepi).
Enantiornitheans: The Strange 'Opposite Birds' of the Cretaceous
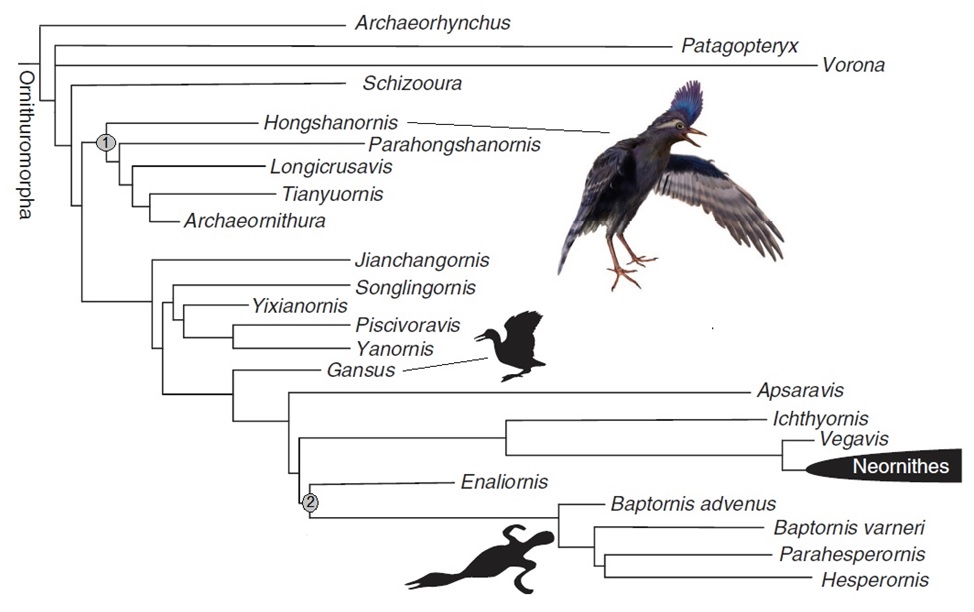
Ornithuromorpha phylogeny. 1) Hongshanornithidae, 2) Hesperornithiformes (Figure modified from Wang et al. 2015).
 \
\
Time-calibrated phylogeny of 198 species of birds. Figure continues on the lower panel from the green arrow at the bottom of the top panel.
The five major, successive, neoavian sister clades are: Strisores (brown), Columbaves (purple), Gruiformes (yellow), Aequorlitornithes (blue), and
Inopinaves (green).
Background colors mark geological periods. Ma, million years ago; Ple, Pleistocene; Pli, Pliocene; Q., Quaternary (From Prum et al. 2015)..
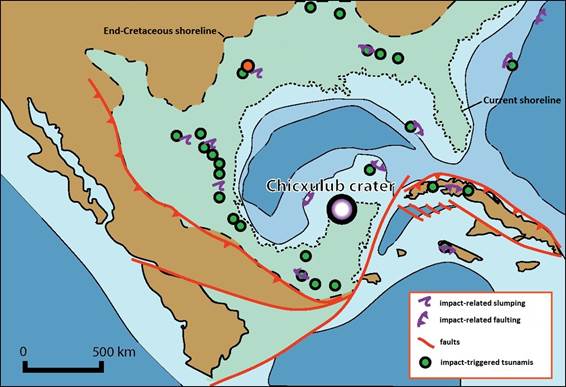
Map of the Gulf of Mexico at the end of the Cretaceous and the location of the Chicxulub crater.
In addition to more local impacts caused by the tsunamis and impact-trigger earthquakes, the particulates and
gases released into the atmosphere would have had global-wide impacts (Figure from Vellekoop et al. 2014).
Video: How did all dinosaurs except birds go extinct?
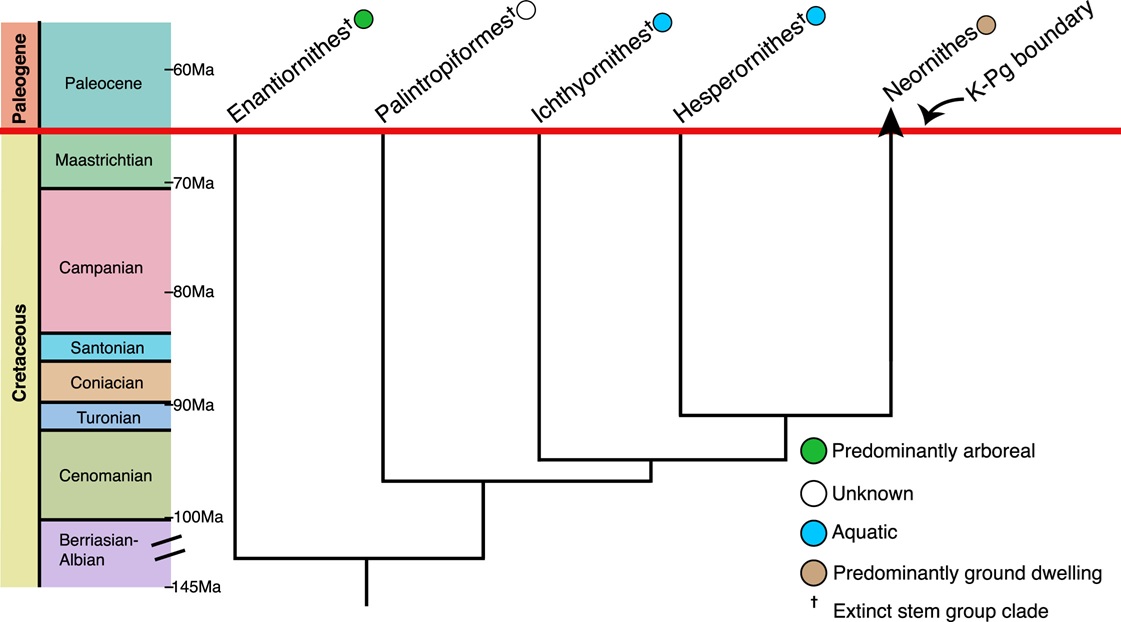
Fossil evidence suggests that all non-neornithine dinosaurs, except the ancestors of present-day birds (Neornithes), perished
in the aftermath of the end-Cretaceous asteroid impact. This includes the Ichthyornithes (Ichthyornis and kin), Hesperornithes (Hesperornis
and kin), Palintropiformes, and the diverse and globally widespread Enantiornithes.
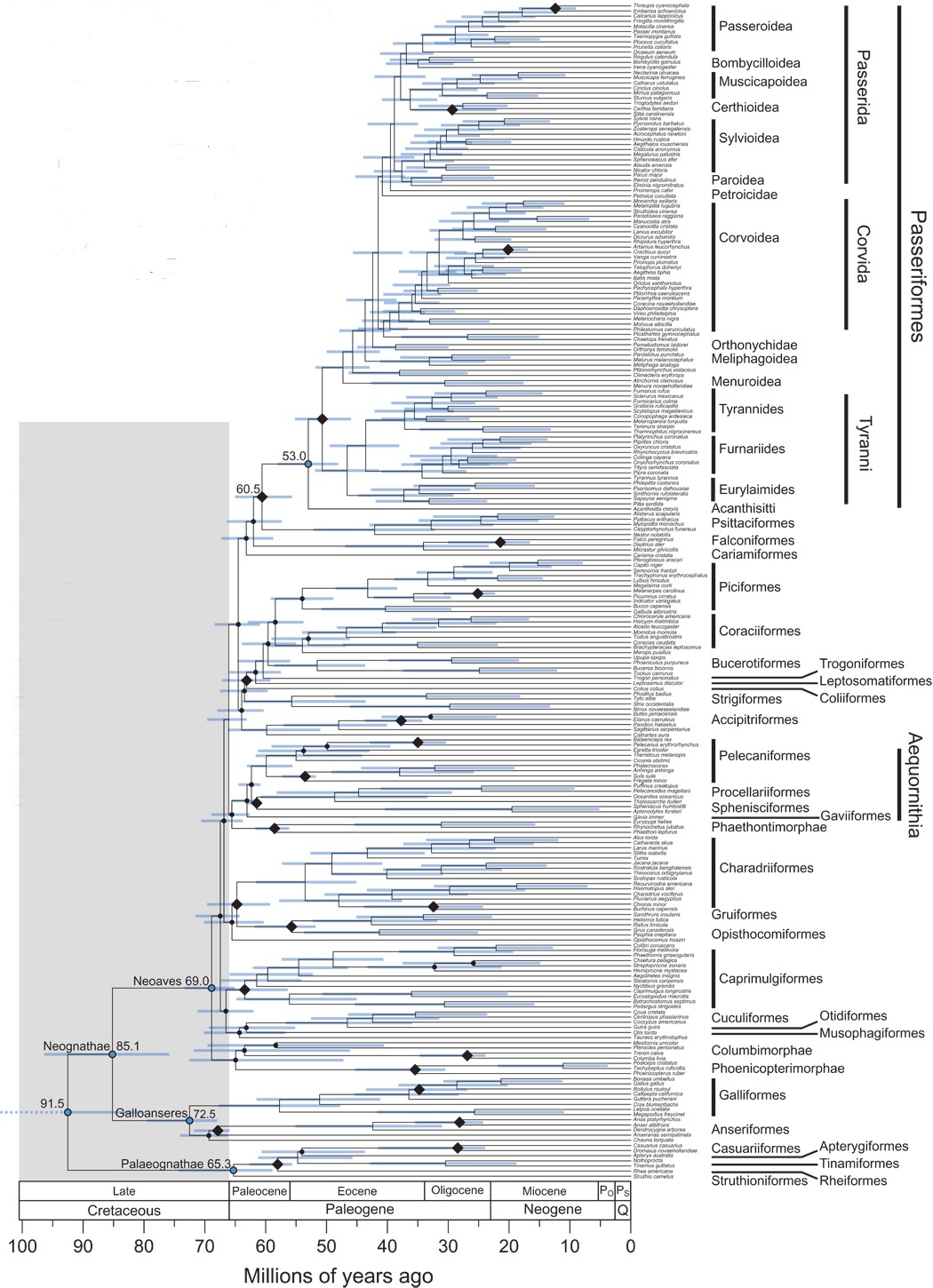
Time tree of modern birds (From: Claramunt and Cracraft 2015).
What’s new in Taxonomy - 2025 Edition

Examples of some functions of feathers. (A) Waterproofing is achieved through feather microstructure. (B) Feathers protect other feathers. In the primary feathers of
Great Crested Flycatchers ‘shading’ can be observed where each feather protects the feather below, and the exposed tips of the feathers are much more faded from solar radiation.
(C) Some birds have stiffened rectrices that aid them in balance during foraging, like this Magellanic Woodpecker. (D) Nestlings of at least two species of cotingas appear to be
Batesian mimics of toxic caterpillars in their juvenile plumage. (E) Feathers used in flight by this Arctic Tern help it fly both through generating and disrupting lift, which helps it
forage acrobatically in the air. (F) Crypsis is achieved in many birds through outline disruption and background matching, as in this Wilson's Snipe. (G) Bristles often cover the nares
or eyes of birds, as in this Common Raven. (H) Brilliant colors of some tropical birds like this Violet-capped Woodnypmh are important for sexual display. (I) The tail feathers of
some hummingbirds create mechanical sounds used in sexual displays. (J) Bright feathers may distract or disorient predators, as in this Royal Flycatcher. (K) Feathers increase a bird's volume
with negligible increase to mass, making most birds very buoyant; conserving energy for water birds. (L) The feathers of some birds have distinctive odors, as in this tangerine-smelling Crested Auklet.
(M) Badges, like the red epaulets on this Red-winged Blackbird are important signals for male–male competition. (N) Many birds appear to engage in evolutionary mimicry of feather pattern
and color, with many unrelated flycatchers converging on the appearance of this Great Kiskadee. (O) Feathers wear in the sun, and coverts protect flight feathers from added wear. When a
birds' wing is folded, the flight feathers are mostly hidden under coverts, as in this Picui Ground-dove. (P) Bright plumages may influence adult behavior, e.g., young American Coots are fed by parents
in proportion to plumage brightness compared to siblings. (Q) Flight serves many purposes in birds, including the ability to pursue and capture aerial prey, like this Olive-sided Flycatcher.
(R) Flight also makes long-distance annual migrations possible, as in this Western Tanager (From: Terrill and Shultz 2023).
How did feathers evolve?
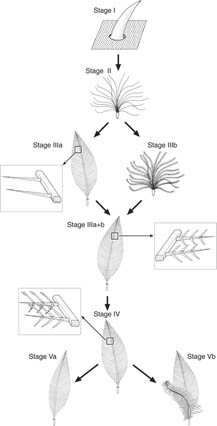
Evolution of feathers -- The evolutionary transition series of feather morphologies predicted by the developmental theory of feather evolution (Prum 1999 ). The model hypothesizes the origin and diversification of feathers proceeded through a series derived evolutionary novelties in developmental mechanisms within the tubular feather germ and follicle:
|
 |
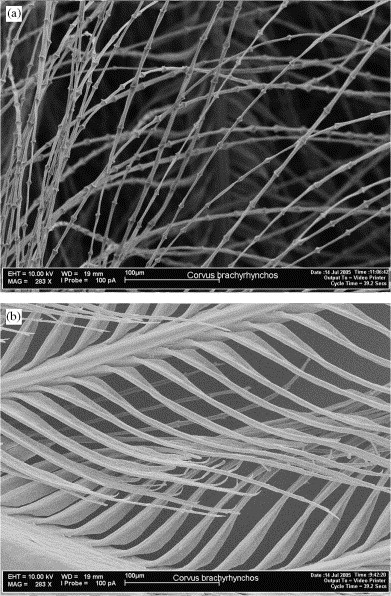
Scanning electron photomicrographs of downy (top) and pennaceous (bottom) barbules
of an American Crow (Corvus brachyrhynchos) (From: Dove et al. 2007).
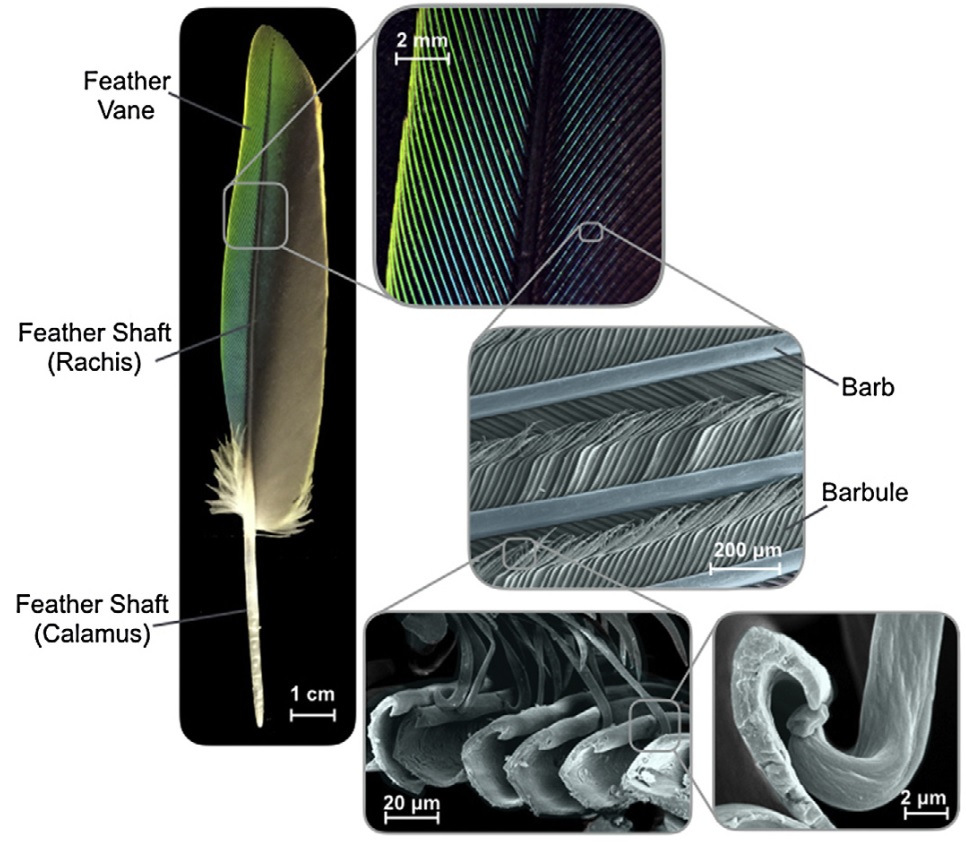
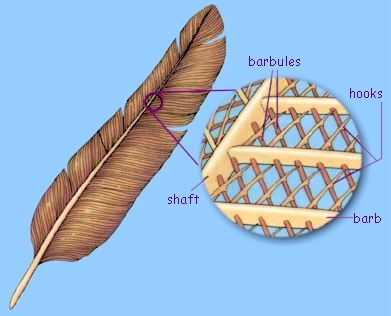
Flight feathers are composed of the feather shaft (rachis and calamus)
and the feather vane (barbs and barbules). Barbs are foam-filled
asymmetrical beams that branch from the rachis and barbules are minute
hooked beams and grooves that branch from barbs to interlock with each
other (Figure from Sullivan et al. 2017).
Basics of Passerine Molt: Theory and Examples
 |
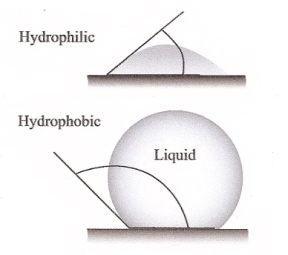 When contact angle increases, interfacial tension between liquid and solid (feather) increases. |
Why do (most) feathers repel water? -- Wettability of solid surfaces with liquids is governed
by the chemical properties and the microstructure of the
surfaces. As far as the microstructure of a surface is
concerned, fine roughness is well-known to enhance the
hydrophobic and hydrophilic properties.
 Blue Whistling-Thrush (Myiophonus caerulea) © Dr. Bakshi Jehangir - www.birdsofkashmir.com
|
Avian Plumage Color (Prum et al. 2003)
-- The
colors of avian plumage are produced by chemical pigments (e.g.,
melanin
or carotenoids) or by nanometer-scale biological structures that
differentially
scatter, or reflect, wavelengths of light. No exclusively blue or
UV-colored
pigments are known in vertebrates, but various carotenoid pigments in
bird
feathers produce UV wavelengths in combination with human-visible
yellow,
orange, or red colors. Ultraviolet structural colors of feathers can be
produced by two types of structures. Primarily iridescent colors are
produced
by arrays of melanin granules in feather barbules. Those structural
colors
are created by coherent scattering, or constructive interference, of
light
waves scattered from the layers of melanin granules in barbules. A few
species of hummingbirds and European Starlings are known to produce UV
hues with coherently scattering melanin arrays in feather barbules. The most commonly distributed UV hues, however, are structural colors produced by light scattering from the spongy medullary layer of feather barbs. To date, primarily UV hues have been documented in the feather barbs of Chalcopsitta cockatoos (Psittacidae) and Myiophonus thrushes (Turdidae). Extensively UV hues with a peak reflectance in the human-visible blue range have been observed in feather barbs of Blue Tits (Parus caeruleus), Bluethroats (Luscinia svecica), and Blue Grosbeak. In addition, Prum et al. (2003) have found extensive UV reflectance from apparently blue feather barbs in many families and orders of birds including motmots (Momotidae), manakins (Pipridae), cotingas (Cotingidae), fairy wrens (Maluridae), bluebirds (Sialia), buntings and others. The structural UV hues of feather barbs, like other barb structural colors, are produced by the keratin air matrix of the spongy medullary layer of the barb ramus. However, the precise physical mechanism by which the human-visible and UV barb colors are produced remains controversial. Analysis of the spongy medullary keratin of UV-colored feather barbs of Myiophonus caerulea by Prum et al. (2003) demonstrated that, in this species, color-producing tissue is substantially nanostructured at the appropriate spatial scale to produce the observed ultraviolet hues by coherent scattering, or constructive interference. |
The iridescent plumage of hummingbirds

The iridescent throat of this Broad-tailed Hummingbird (Selasphorus platycercus)
changes dramatically in appearance from
black to magenta depending on the viewing angle and/or the angle of illumination. The same
individual
is shown in all images
(Photos by Mary Caswell Stoddard and from Cuthill et al. 2017).

Color patterns of feathers. (A) Representative patterns within feathers. (B) Some other basic patterns such as bars, circles, and spots.
(C) There are also, of course, color patterns at the level of the entire body (From: Yu et al. 2004).
Adventures in Bird Molt: Finding fun in a forbidding yet fundamental process. By Peter Pyle.
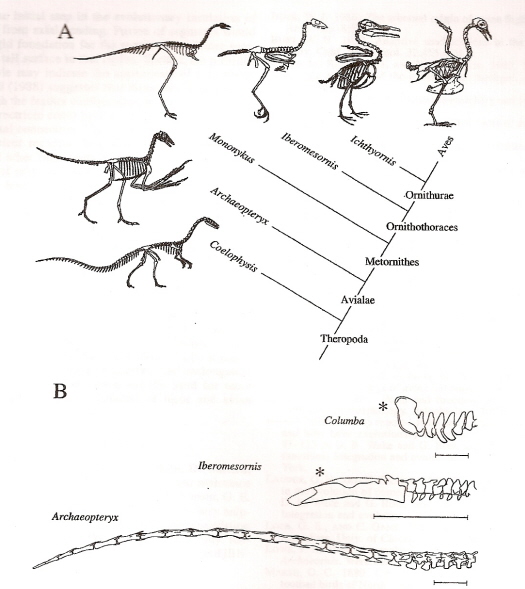
A. The relationship between select theropods and tail reduction in bird evolution.
B. Evolution of short-tailed birds exemplified by Archaeopteryx, Iberomesornis, and Columba (pigeon)
tail vertebrae. Note the reduction in number of vertebrae and centrum (body) length. Both Iberomesornis
and Columba possess a pygostyle (asterisk). Scale bar = 2 cm (Gatesy and Dial 1996).

Morphological diversity of bird bills. Top row (left to right): Mrs. Gould's Sunbird, Long-billed Wren-Babbler, Red-headed Trogon,
Common Green-Magpie, Horned Puffin. Second row (left to right): Russet Sparrow, Steppe Eagle, Asian Openbill,
Silver-breasted Broadbill, Greater Flamingo. Third row (left to right): Pied Avocet, Common Raven, Crested Serpent-Eagle ,
Asian Emerald Dove,Shy Albatross. Fourth row (left to right): Blue-and-yellow Macaw, Mountain Tailorbird, Black-breasted Parrotbill, Sikkim Wedge-billed Babbler,
Sri Lanka Frogmouth. Bottom left: Eurasian Spoonbill. Fifth row (left to right): Black-crowned Scimitar-Babbler, Gold-naped Finch,
Kea. Sixth row (left to right): Slender-billed Scimitar-Babbler, Southern Royal Albatross, Common Hoopoe, Red Junglefowl.
Bottom right: Spot-billed Pelican (From: Krishnan 2023).
Golden Eagle skull
Source: http://www.azdrybones.com/birds.htm
Bird beaks
The loss of teeth in birds (from Louchart and Viriot 2011) -- The Cenozoic bird fossil record (65.5 million years ago to present) contains only toothless Neornithes. By contrast, most Mesozoic birds (146 to 65.5 million years ago) had teeth. Thus, edentulism (complete loss of teeth) in Neornithes occurred between about 125 and 65.5 million years ago. The acquisition of a muscular gizzard and of a rhamphotheca appear to have been crucial in allowing edentulism and making it viable. Food is stored in the crop, and hence continuously available even outside feeding activities. The muscular gizzard efficiently processes this food, allowing the continuous provision of abundant nutrients necessary for the high metabolic demands of flight. Together with many morphological changes, such as lightening of the skeleton, skeletal structure reinforcements and fusions, and displacement of the center of gravity, higher metabolic rates allowed the improvement and diversification of sustained powered flight. Homeothermy and sustained powered flight arose in an indirect link with the whole process of tooth loss in birds, and with other innovations.
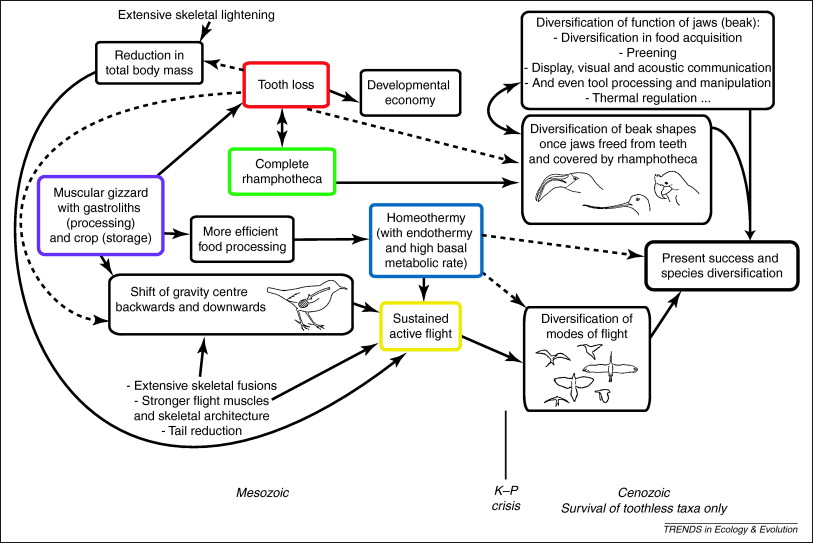
Proposed evolutionary interactions related to the loss of teeth in birds. Several major morphological, physiological and behavioral innovations favored or made possible (arrows) the evolution of other innovations in a complex way: some facilitated edentulism in birds, whereas others led to avian evolutionary success following, and despite, tooth loss, as the Aves are the most speciose class of extant tetrapods. Dashed arrows represent less obvious influences. The horizontal distribution of events reflects approximately their relative temporal occurrences, when known, although some cannot be assigned to a well-defined relative placement.
The loss of teeth in birds allowed for unprecedented diversification of rhamphothecae in terms of size and shape. The diversity in beak shapes and functions in extant birds exceeds by far that observed in the jaws or snout of all other tetrapods, and involves slender or light architectures, extremely varied shapes and curvatures, and specialized kineses that would have been impossible with dentition. By contrast, Mesozoic birds that retained teeth show only a limited diversity of shapes of the snout or incipient beak. The evolution of diverse extreme beak shapes was completed during the first half of the Cenozoic, following tooth loss, in pelicans, stork-like birds, duck-like and flamingo-like taxa, birds of prey, wide-gaped and short-beaked aerial insectivores, and even hummingbirds. The rhamphotheca proves at least as efficient as teeth for food acquisition, whether it is smooth or serrated. Beaks also took on additional functions secondarily, such as feeding young, preening, grooming, courtship and display, communication, and even tool manufacture and manipulation. Such functions have probably contributed to the success of the Neornithes.
>
In a mangrove forest in Miocence South America, a Devincenzia pozzi — an over two-meter tall phorusrhacid — has brought
prey (the giant rodent
Cardiotherium, an extinct relative of capybaras) to its chicks. Note the massive legs and hooked beak (artwork
by Gabriel Ugueto) (From: Maderspacher 2022).
Source: sped2work.tripod.com/evidence.html
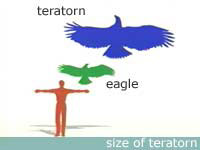
A South American 'terror bird' (a Phorusrhacid).
Terror Birds: This extinct group of predatory, flightless birds dominated South America from
~62 million years ago to the mid-Pleistocene. The largest known terror-bird species grew to nearly 10 feet
(3 meters) tall and
weighed 1,100 pounds (500 kilograms).
|
|
 Ostrich |
Why so many small birds? --
Birds range about 41,000-fold in body size from the tiny 2-g Bee Hummingbird (Calypte helenae) to
Ostriches (Struthio camelus) that can weigh over 100 kg. However, more than half of all bird species weigh less than 38 g. Generally, small-bodied
species are also more abundant (more individuals) than large-bodied species. The same patterns have been documented for several groups of
organisms, e.g., snakes and mammals, which suggests that there is a general reason why there are so many small species.
The very unequal distribution of
body sizes in evolutionary lineages could be the outcome of biased evolution, with natural selection favoring small size.
This hypothesis has received a lot of
discussion in the recent literature, but has thus far not has been convincingly demonstrated.
Another possibility is that small-bodied species speciate faster.
However, statistical analyses accounting for historical relatedness of present-day species indicate no relation between body size and the rate of speciation.
Finally, instead of little by little, the dominance of small species may have arisen suddenly, when approximately 65 million years ago (presumably) a large
meteorite
hit the earth, causing mass extinctions. However, analysis of body sizes and genetic differences of extant species reveals that while avian species
numbers
were approximately halved, the catastrophe affected small and large species equally. Thus, the reason why most species are small does not seem to
be
due to
differential
rates of speciation or extinction. Rather, the cause appears to be in the tempo and mode of evolution. Analyses of the body sizes of living
birds
suggest that most differences in body size between species arise at the moment of speciation. Differences between small-bodied species are smaller
than
between large-bodied species
and this difference probably also has its origin at the moment of speciation. Consequently, groups of small species stay
small,
whereas groups of large species are more variable in body size, so that in the end most species are small (Bokma 2002, Bokma 2004).
Despite variation in size, all living birds exhibit a remarkable similarity because of their (or their ancestor's) adaptations for flight. The success of birds, as a group, is in large part due to this ability to fly! Flight is, however, demanding and the bird body shows several modifications for this mode of locomotion, including lightness, streamlining (see European Starling below), strength (rigid skeleton and strong, efficient muscles), and efficient energy utilization
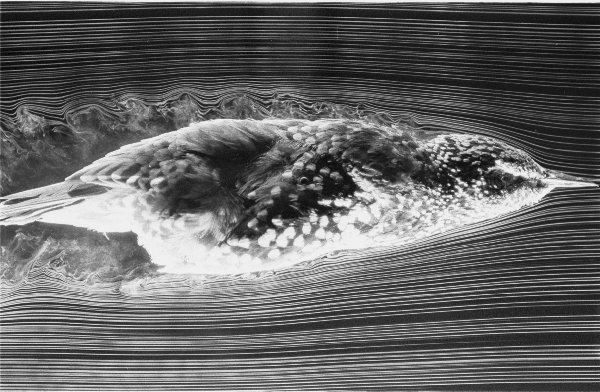
A European Starling in a wind tunnel with an airflow
of
9 m/s visualized using the smoke-wire technique.
(Source: http://www.biology.leeds.ac.uk/staff/jmvr/Flight/fvbzwjm.htm)\
Avian skeleton: adaptations for flight
The skeleton of birds shows numerous modifications for the
demands
of flight:

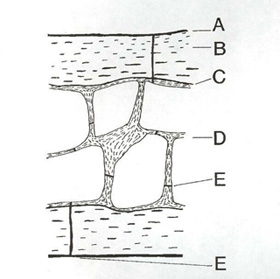
Schematic cross-section through a bird bone.
A - periosteal surface, B - lamellar cortical layer,
C - endiosteal surface, D - trabecular layer,
E - pores/pneumatic openings/blood vessel openings
(From: Davis 1998).
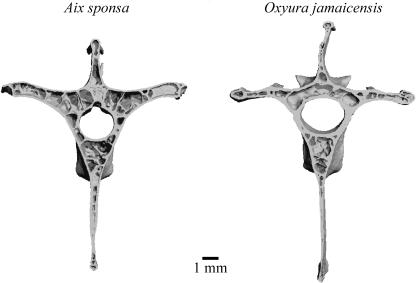
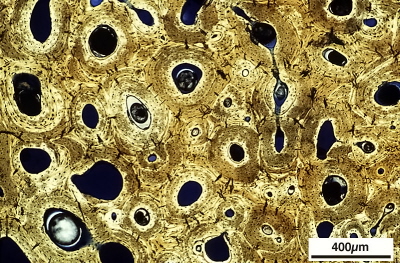
Vertebrae of a Wood Duck (Aix sponsa) and a Ruddy Duck (Oxyura jamaicensis) showing the outer layers of compact cortical bone surrounding the trabecular bone. The vertebrae of Ruddy Ducks have more compact bone and less trabecular bone. Ruddy Ducks are diving ducks whereas Wood Ducks are dabbling ducks that forage at or near the water’s surface. More compact bone and less trabecular bone makes bones, and diving ducks, heavier and, therefore, makes them more efficient divers (Figure from Fajardo et al. 2007).

Diversity of avian cervical morphology. (a) Variation in number of cervical vertebrae for 112 species of birds. Branches are coloured based on the number of cervical vertebrae. (b–e) Variation in the cervical region of the vertebral columns of (b) Kakapo, (b) Osprey, (c) Anhinga, and (d) Taiga Bean-Goose. (e) shows the colors of vertebrae C2 (blue), a vertebrae located at the midpoint of the cervical column (pink), and the last cervical vertebrae (gold) (From: Marek 2023).
.
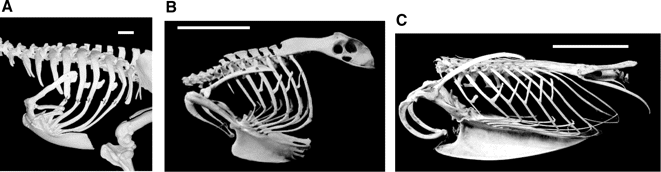
Representative skeletons showing the morphological differences in the rib cage associated with different forms of locomotion in (A) a walking species, Cassowary (Casuaris casuaris); (B) a non-specialist, Eagle Owl (Bubo bubo); and (C) a diving species, Razorbill (Alca torda). Uncinate processes are short in walking species, of intermediate length in non-specialists and long in diving species. In all photographs cranial is to the left; scale bar, 5 cm.
Functional significance of the uncinate processes in birds -- Uncinate processes are bony projections that extend from the vertebral ribs of most extant birds. In 1935, Zimmer (1935) postulated that the uncinate processes played some role during inspiration. Other hypotheses have linked these processes with stiffening or strengthening the rib cage or providing attachment sites for muscles stabilizing the shoulder. Recent electromyographic studies of Giant Canada Geese confirmed Zimmer's hypothesis by demonstrating that these processes are integral component of the ventilatory mechanics of birds being involved in both inspiration and expiration (Codd et al. 2005). The processes are associated with fleshy parts of the Mm. intercostales externi, the Mm. appendicocostales that originates from the proximal edge of the uncinate and inserts onto the following vertebral rib. The Mm. appendicocostales is active during inspiration in Giant Canada Geese, suggesting the processes facilitate the craniad movement of the ribs, which would in turn move the sternum ventrally. The base of the uncinate processes serves as a brace for the insertions of the `finger-like' projections of the M. externus obliquus abdominus that pull the sternum dorsally during expiration. Given that the processes provide attachment sites for these important respiratory muscles, any change in uncinate morphology may have a significant effect on ventilation.
Tickle et al. (2007) derived a model demonstrating that uncinates act as levers that improve the mechanical advantage for the forward rotation of the dorsal ribs and therefore lowering of the sternum during respiration. The length of these processes is functionally important; longer uncinate processes increasing the mechanical advantage of the Mm. appendicocostales muscle during inspiration. Morphological studies of four bird species showed that the uncinate process increased the mechanical advantage by factors of 2–4. An examination of variation in skeletal parameters in birds with different primary modes of locomotion (non-specialists, walking and diving) revealed that uncinate length is more similar in birds that have the same functional constraint, i.e. specialization to a locomotor mode. Uncinate processes are short in walking birds, long in diving species and of intermediate length in non-specialist birds. These results demonstrate that differences in the breathing mechanics of birds may be linked to the morphological adaptations of the ribs and rib cage associated with different modes of locomotion.
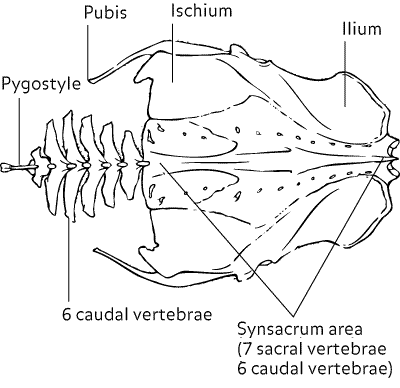
Bird tail bones - Pygostyle
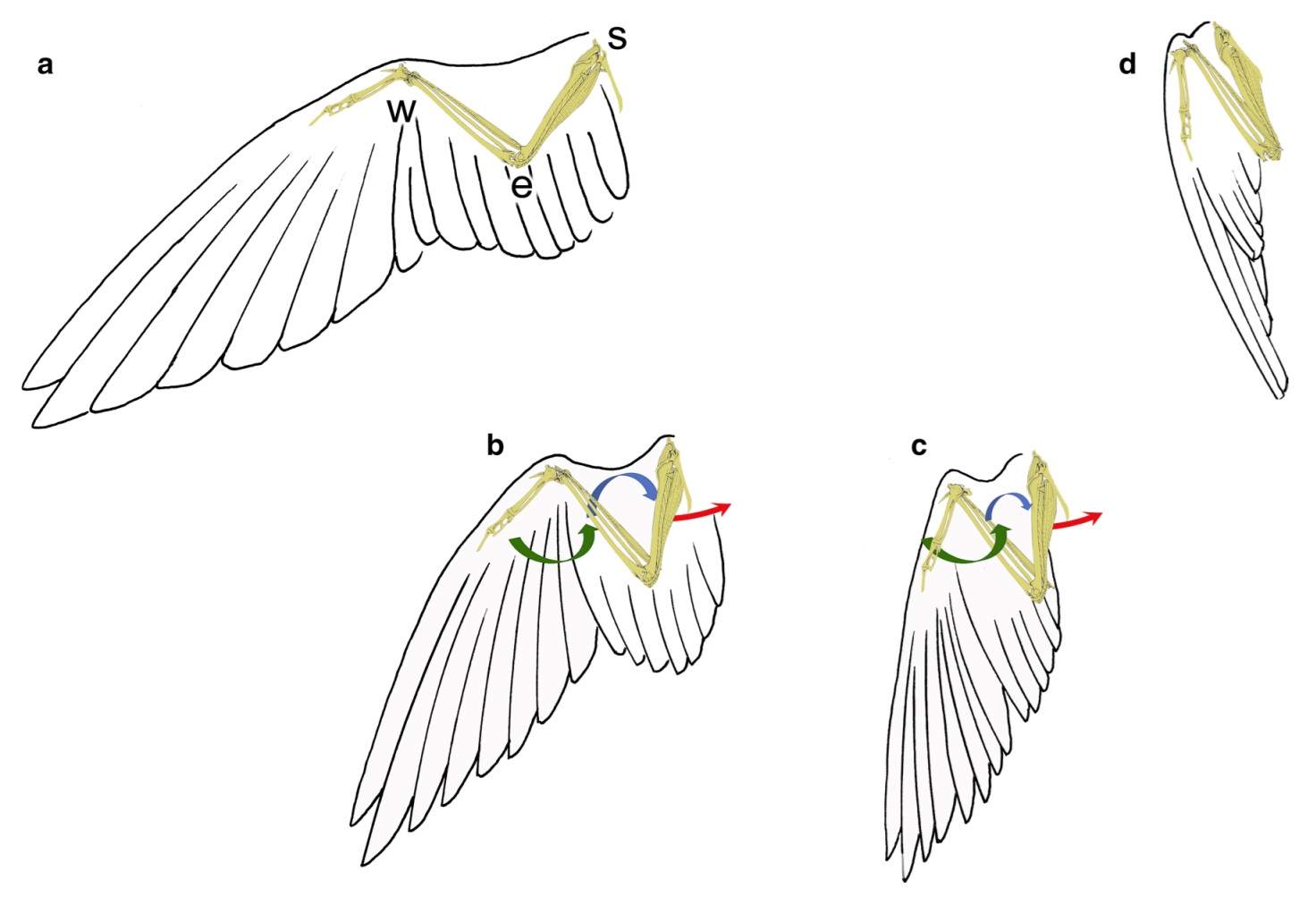
Actions necessary to produce and maintain the folded avian wing posture. Arrows indicate
flexion movements at the wrist (green) and elbow (blue), and the adduction movement of the humerus (red).
a–d Represent sequential positions of the closing wing. e, elbow joint; s, shoulder joint; w, wrist joint (From: Meyers et al. 2019).
The forelimbs of another flying vertebrate (lesser short-nosed fruit bat, Cynopterus brachyotis)
are very different from those of birds (and now-extinct reptiles, pterosaurs, could also fly).
Egyptian fruit bat - slow motion

Aerodynamic efficiency: birds vs. bats -- Flight is one of the energetically most costly activities in the animal kingdom, suggesting that natural selection should work to optimize flight performance. The similar size and flight speed of birds and bats may therefore suggest convergent aerodynamic performance; alternatively, flight performance could be restricted by phylogenetic constraints. Muijres et al. (2012) tested which of these scenarios fit to two measures of aerodynamic flight efficiency in two passerine bird species (Pied Flycatcher and Blackcap) and two New World leaf-nosed bat species. Using time-resolved particle image velocimetry measurements of the wake of the animals flying in a wind tunnel, the span efficiency, a metric for the efficiency of generating lift, and the lift-to-drag ratio, a metric for mechanical energetic flight efficiency, were derived. Birds significantly outperformed the bats in both metrics, likely due to variation in aerodynamic function of body and wing upstroke: Bird bodies generated relatively more lift than bat bodies, resulting in a more uniform spanwise lift distribution and higher span efficiency. A likely explanation would be that the bat ears and nose leaf, associated with echolocation, disturb the flow over the body. During the upstroke, the birds retract their wings to make them aerodynamically inactive, whereas the membranous bat wings generate thrust and negative lift. Despite the differences in performance, the wake morphology of both birds and bats resemble the optimal wake for their respective lift-to-drag ratio regimes. This suggests that evolution has optimized performance relative to the respective conditions of birds and bats, but that maximum performance is possibly limited by phylogenetic constraints. Although ecological differences between birds and bats are subjected to many conspiring variables, the different aerodynamic flight efficiency for birds and bats may help explain why birds typically fly faster, migrate more frequently, and migrate longer distances than bats.
| Bird embryos have 5 fingers -- The developmental origin of digits in the wings of birds has been hotly debated for more than a century. Larsson and Wagner (2002) have shown unequivocally that five digits are present during the early development of chickens. The earliest stage of digits is a condensation of mesenchymal cells and digit I is, thus, transiently present during development. This establishes that three digits in the wings of birds are digits II–IV. However, theropod dinosaurs are assumed to have had digits I–III. Feduccia & Nowicki (2002) claim that for this reason, a descent of birds from theropods is impossible and that instead, birds are descended from archosaurs other than dinosaurs (e.g., thecodonts). Galis et al. (2002) believe it improbable that the multitude of shared characters between theropods and birds are the result of convergence. That leaves three possible scenarios: (1) birds descending from archosaurs other than dinosaurs, which cannot satisfactorily explain the many similarities between birds and theropods; (2) the 'frame shift hypothesis' [theropod ancestors of birds initially had digits I–III and, before the origin of birds, a shift occurred such that digits II–IV developed with identities I–III; Wagner and Gauthier (1999)] for which there is as yet no adaptive significance that would overcome the evolutionary constraint; and (3) birds descending from theropods with digits II–IV, which is the most parsimonious evolutionary transition scenario but for which there is as yet no fossil evidence. | 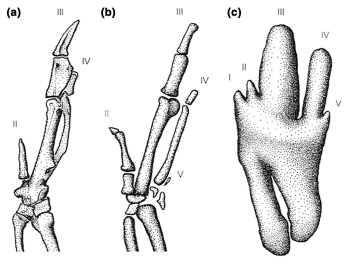 Developmental stages of chick wings in dorsal view. (a) Adult wing with three ossified digits. (b) Stage 35 embryo with four chondrified digits. (c) Stage 29 embryo with five mesenchymal digits (From: Galis et al. 2002). |

Leg bones of six different species of birds showing the variation in the bone length proportions. From left to right:
Red-throated Loon, African Penguin, Common Wood-Pigeon, Black-billed Magpie, European Robin, and Greater Flamingo (From: Zeffer et al. 2003).
Pelvic girdle and hindlimb anatomy
Crouching turkey, hidden dragon
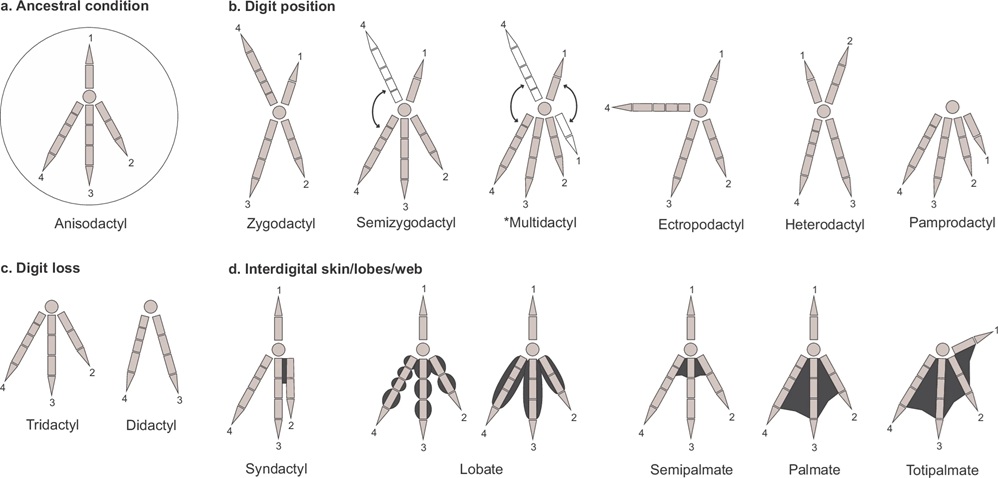
Foot types of birds. (a) The ancestral foot type, from which the other foot types evolved. Classification based on: (b) the
positional arrangement of digits and ability to rotate and change the position of some of them, (c) the secondary loss of digits
and the remaining number of digits, and (d) the presence and extent of skin, lobes or webbing between digits. Multidactyl =
birds able to rotate both the first and fourth digits to either a cranial or a caudal position (e.g., Speckled Mousebirds, Colius
striatus) (From: Carril et al. 2024).
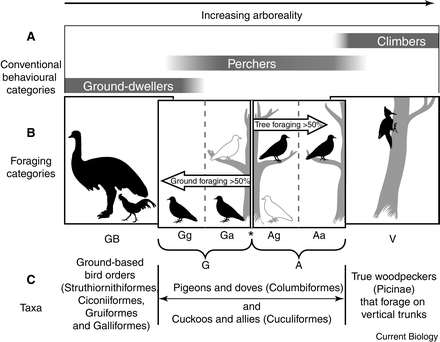
Avian toe claws -- Glen and Bennett (2007) placed birds into six categories (GB, Gg, Ga, Ag, Aa and V)
based the degree of ground or tree foraging; GB = ‘ground-based’ birds, limited to
foraging on
the ground; Gg = ‘dedicated ground foragers’; Ga = ‘predominantly ground foragers’; Ag = ‘predominantly arboreal
foragers’; Aa = ‘dedicated arboreal foragers’; V = ‘vertical surface foragers’. Analysis of the toe claws of 249 species of birds
revealed that claw
curvature increases as tree foraging becomes more predominant.
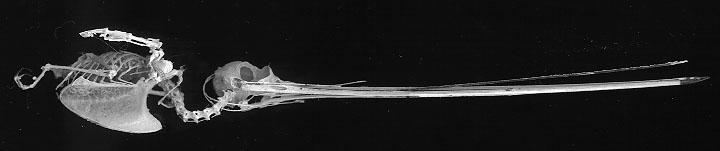
Sword-billed
Hummingbird (Ensifera ensifera) skeleton
(Used with permission of Dennis Paulson, Director,
Slater
Museum of Natural History)
Sword-billed Hummingbird
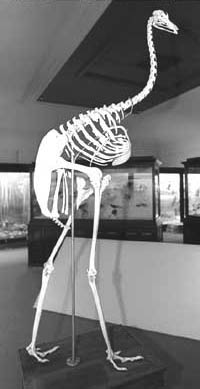
Rhea (Rhea americana) skeleton
Source: http://www.uiowa.edu/~fyi/issues2000/03232001/bird.html
Ratite & carinate birds
Greater Rhea running (about 47 mph, or 70 km/h)
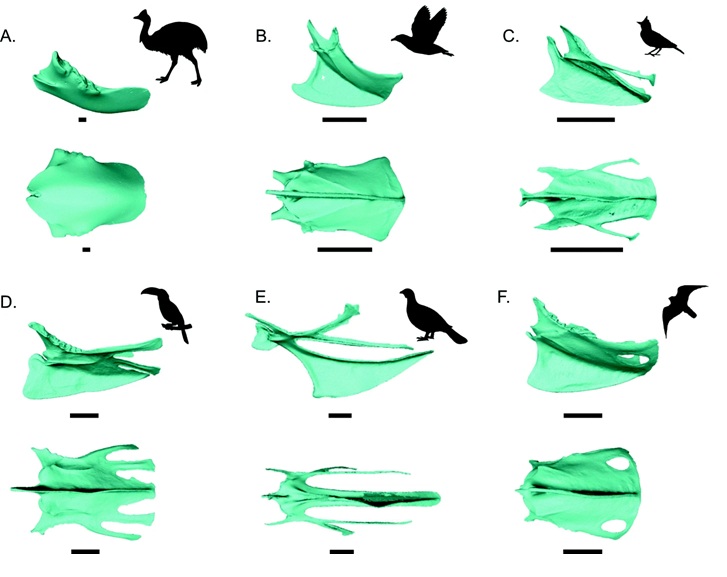
Overview of sternal variation. Sterna shown in lateral view (above) and ventral view (below). A -
Southern Cassowary, B - Leach’s Storm-Petrel), C - Red-capped Lark,
D - Yellow-throated Toucan, E - Chukar, and F - American Kestrel. Scale bars: 10 mm (From Lowi-Merri et al. 2021).
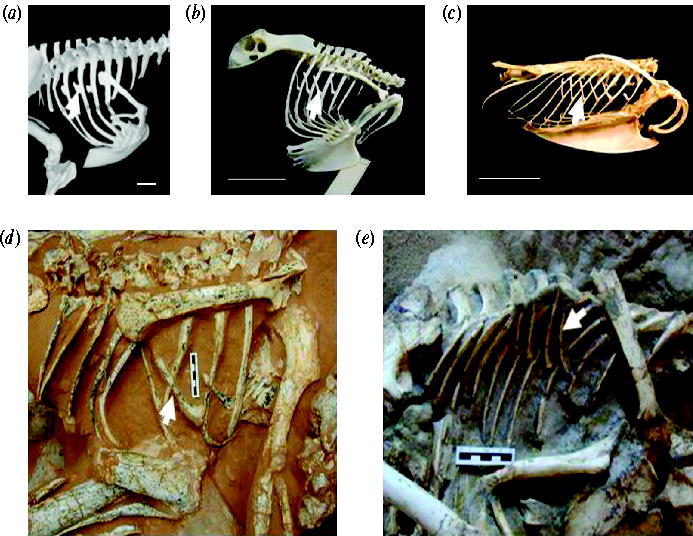
Uncinate processes (arrows) of (a) a running bird, the Cassowary (Casuaris casuaris), (b) a flying bird, the Eagle Owl (Bubo bubo), (c) a diving bird, the Razorbill (Alca torda); analysis indicates that uncinate processes are shorter in running, long in diving, and intermediate in all other birds, (d) Oviraptor philoceratops, (e) Velociraptor mongoliensis. Anterior is to the right in all figures. Scale bars, 5 cm.
Dinosaurs and birds share uncinate processes -- In 1868, Thomas Huxley first proposed that dinosaurs were the direct ancestors of birds and subsequent analyses have identified a suite of ‘avian’ characteristics in theropod dinosaurs. Ossified uncinate processes are found in most species of extant birds and also occur in extinct non-avian maniraptoran dinosaurs. Their presence in these dinosaurs represents another morphological character linking them to Aves, and further supports the presence of an avian-like air-sac respiratory system in theropod dinosaurs, prior to the evolution of flight. Codd et al. (2007) conducted a phylogenetic analysis of the presence of uncinate processes in Aves and non-avian maniraptoran dinosaurs and found they were homologous structures. Furthermore, recent work on Canada Geese has demonstrated that uncinate processes are integral to the mechanics of avian ventilation, facilitating both inspiration and expiration. In extant birds, uncinate processes function to increase the mechanical advantage for movements of the ribs and sternum during respiration. The study by Codd et al. (2007) presents a mechanism whereby uncinate processes, in conjunction with lateral and ventral movements of the sternum and gastral basket, affected avian-like breathing mechanics in extinct non-avian maniraptoran dinosaurs.
The muscles of birds have also been modified by natural selection to meet the demands of flight:
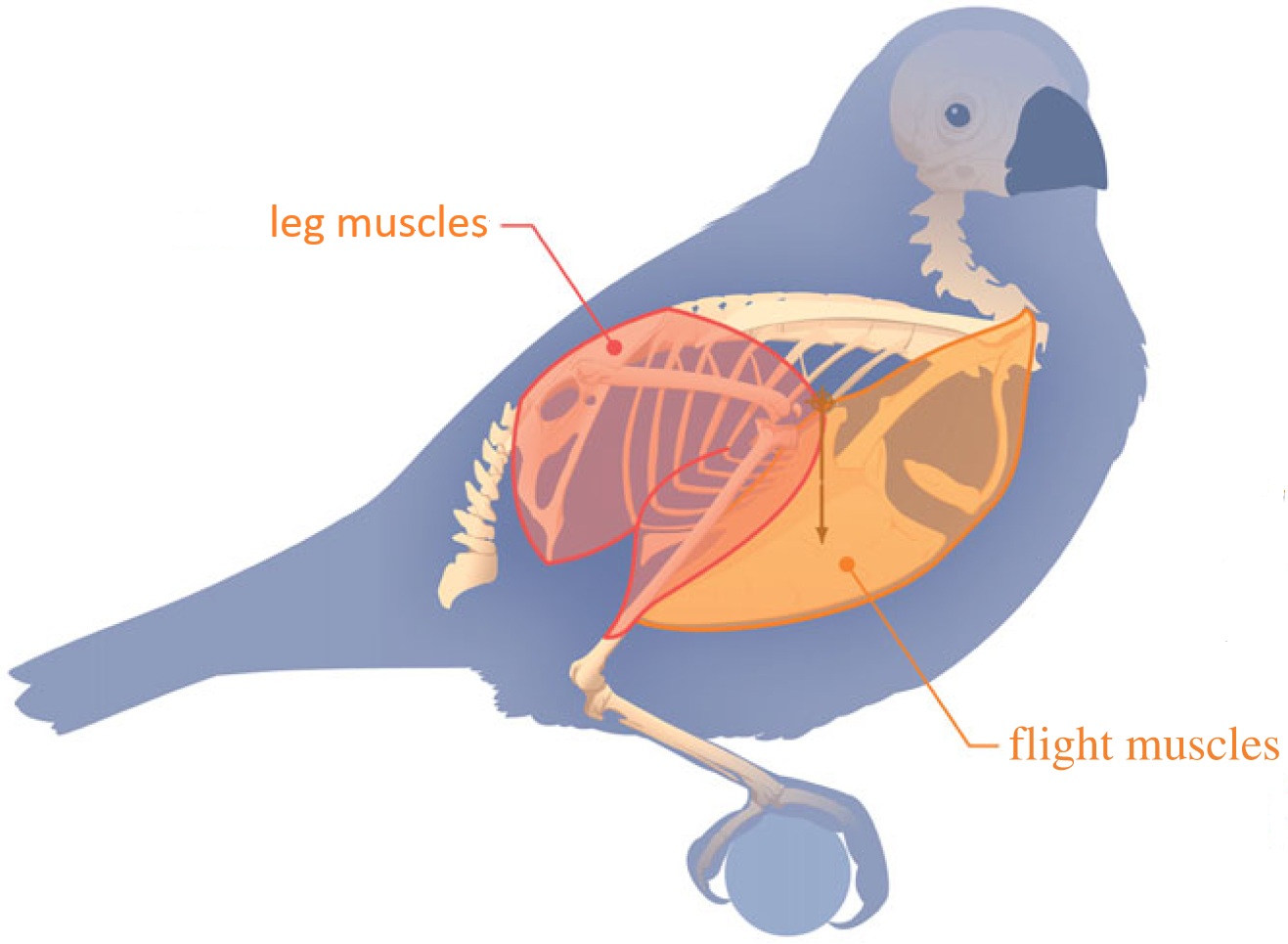
(From: Abourachid et al. 2023)
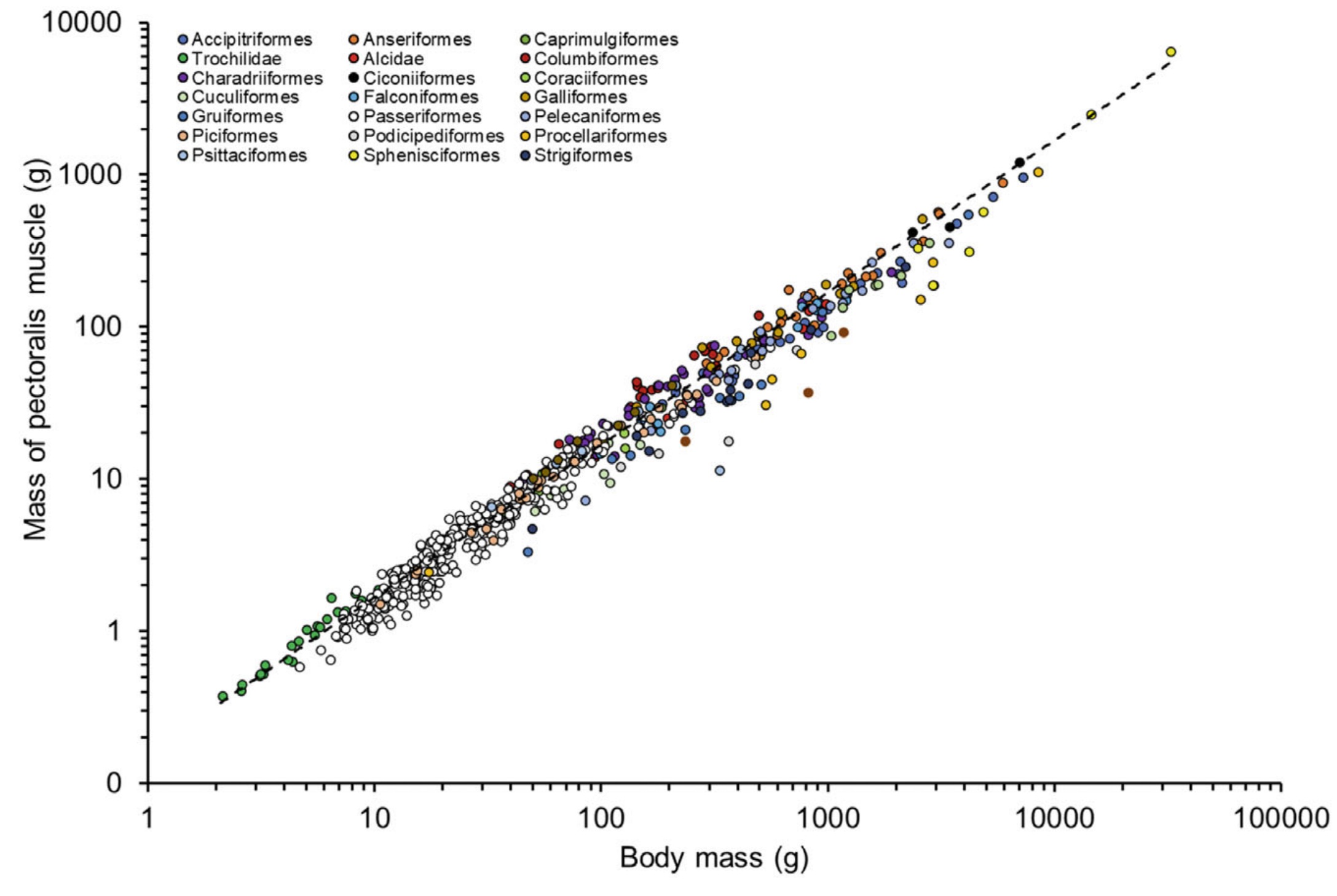
Relationship between body mass and the mass of the pectoralis muscle in various orders of birds. Note the log10 scale on both
axes.
Dashed line indicates the phylogenetically corrected regression estimate through all of the data. This relationship was maintained when
taxonomic grouping was included in the model, with a significant effect
of order. Auks (Alcidae) and pigeons (Columbiformes) tended to be
above the line
through all data, with penguins (Sphenisciformes) and tinamous (Tinamiformes) generally lying below the line (From: Deeming 2023).
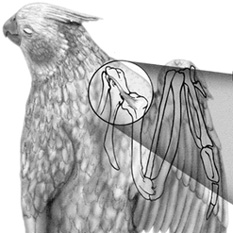
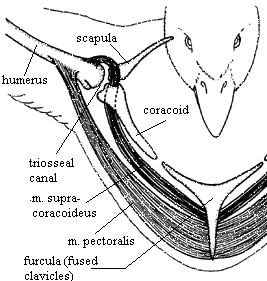
Tendon of the supracoracoideus passing through the
foramen triosseum and inserting on the humerus
(From: Degernes and Feduccia 2001).
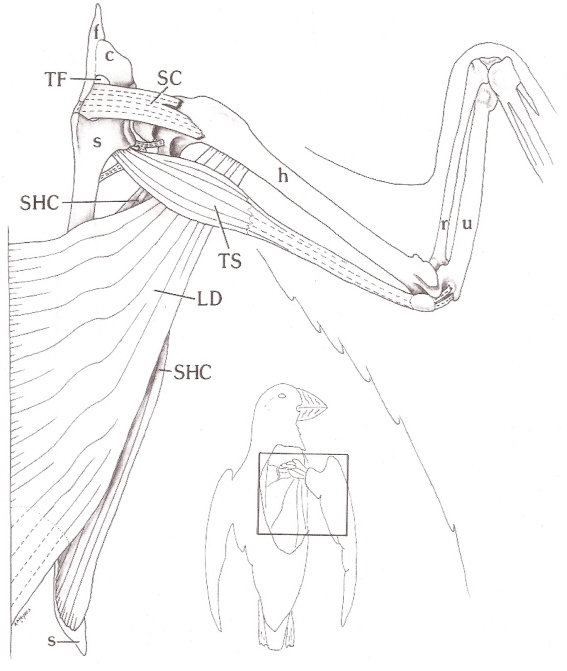
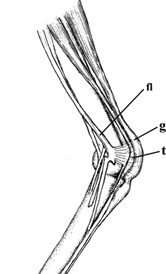
Right wing of an Atlantic Puffin. c, coracoid; f, furcula, h, humerus, LD, latissimus dorsi muscle;
r, radius; s, scapula; SC, supracoracoideus tendon; SHC, scapulohumeralis caudalis muscle;
st, sternum; TF, triosseal foramen or canal; TS, triceps scapularis muscle; u, ulna
(From: Kovacs and Meyers 2000).
Migratory shorebirds
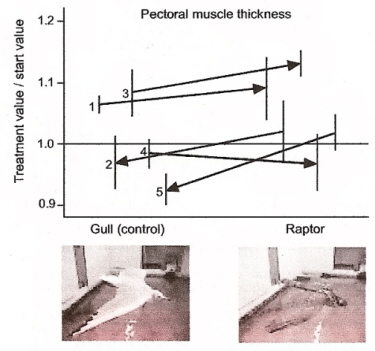
Changes in pectoral muscle size due to simulated raptor attack compared with control treatment (gull),
shown for each of five trials. The direction of each arrow reflects the treatment order (predator after gull or vice versa).
Ruddy Turnstones build pectoral muscle after raptor scares -- To cope with changes in the environment, organisms not only show behavioral but also phenotypic adjustments. This is well established for the digestive tract. Van den Hout et al. (2006) described the first case of birds adjusting their flight machinery in response to predation risk. In an indoor experiment, Ruddy Turnstones (Arenaria interpres) were subjected to an unpredictable daily appearance of either a raptor or a small gull (as a control). Ruddy Turnstones experiencing threat induced by a flying raptor model, longer than after similar passage by the gull model, refrained from feeding after this disturbance. Pectoral muscle mass, but not lean mass, responded in a course of a few days to changes in the perceived threat of predation. Pectoral muscle mass increased after raptor scares. Taking the small increases in body mass into account, pectoral muscle mass was 3.6% higher than aerodynamically predicted for constant flight performance. This demonstrates that perceived risk factors may directly affect organ size.
Ruddy Turnstones - flock flying at regular speed, then in slow motion
 |
 |
 |
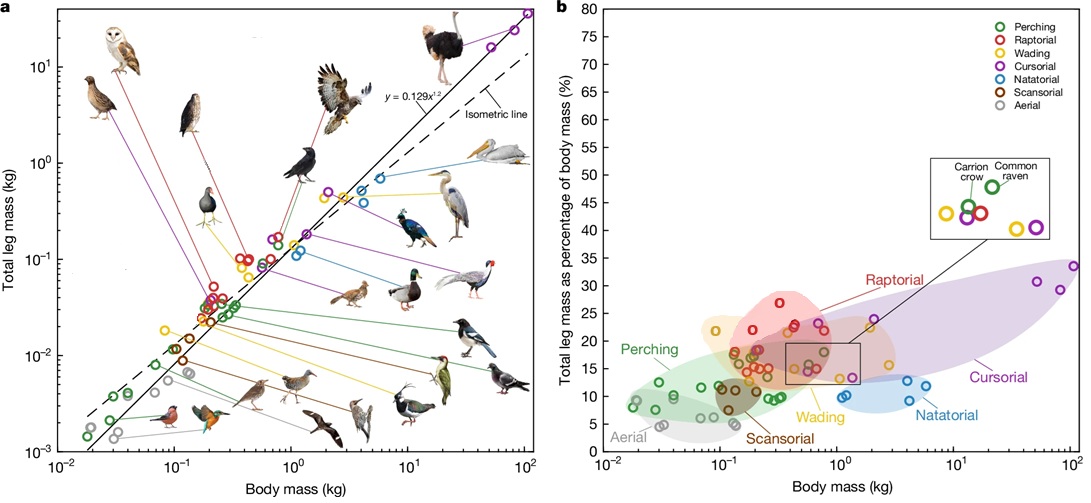
(a) Total leg mass (for two legs, i.e., bones, muscles, tendons, etc.) relative to total body mass among different species of birds. The black solid line represents the trend line of the relationship between the total leg mass
to total body mass in birds The dashed line is the line of isometry. Images of birds are not drawn to scale. (b) Total leg mass as a percentage of body mass. Note that
cursorial birds, like Ostriches, and swimming/diving birds (i.e., Natatorial), like pelicans, have relatively greater leg mass than other species of birds. In addition, raptors and wading
birds generally have greater leg mass than perching birds and aerial (e.g., swifts) birds (From: Shin et al. 2024).
Summary - Avian anatomical adaptations for flight:
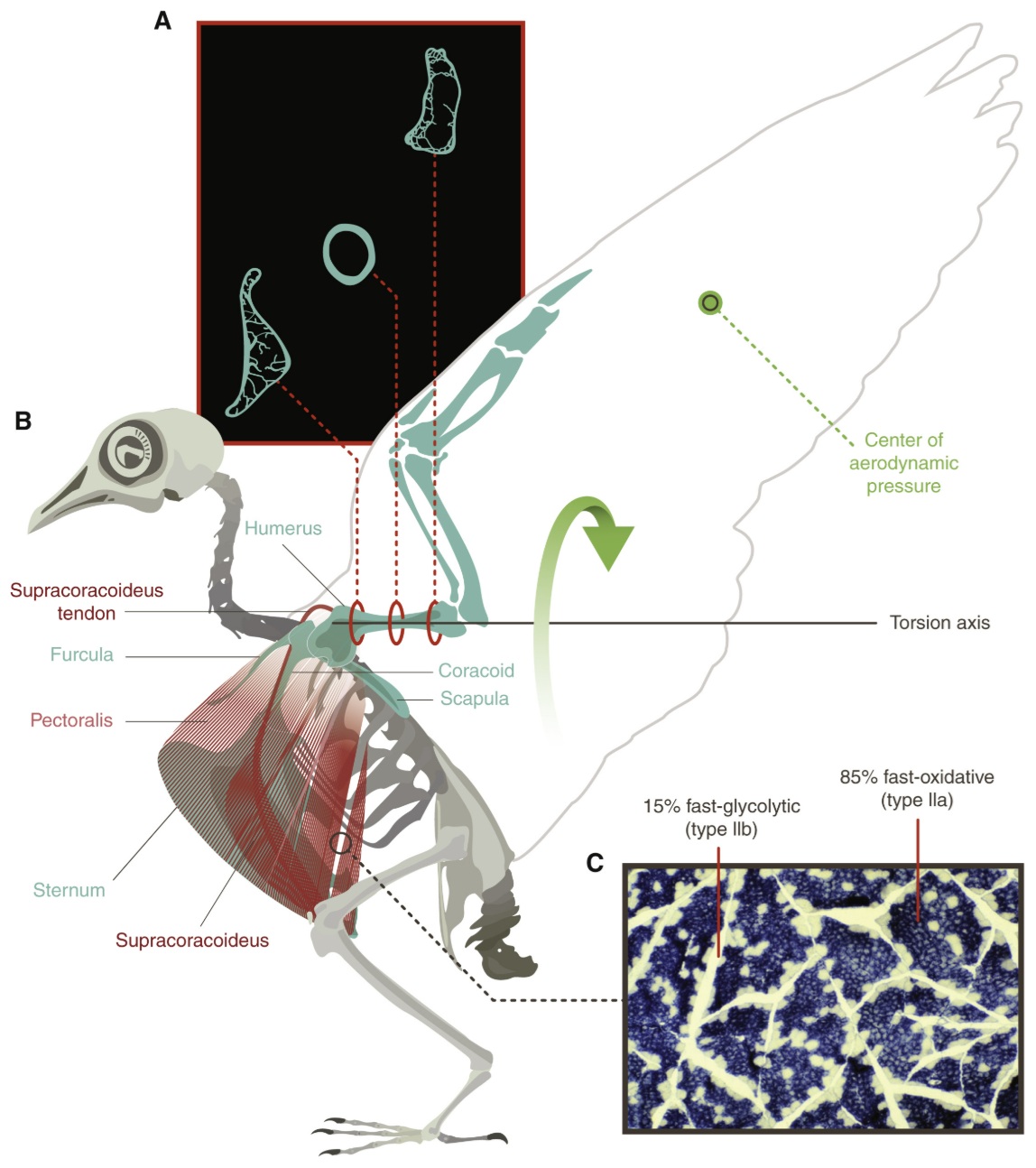
Avian musculoskeletal adaptations for flight.
(A) The forelimb, shoulder and trunk skeleton of birds
underwent several substantial evolutionary modifications to facilitate an effective flapping wing stroke
powered by large flight muscles: the main downstroke pectoralis muscle (light red), and the main upstroke
supracoracoideus muscle (darker red). The ventral keel of the sternum is enlarged, allowing for increased
size of the pectoralis and the underlying supracoracoideus, which acts via its tendon and by means of a
skeletal pulley at the shoulder to elevate the wing. The shoulder joint is supported by robust coracoids
that articulate with the scapula above and sternum below, along with joint ligaments and anteriorly by the
furcula (wishbone). The skeleton is lightweight by (B) many of the wing bones being air-filled and with
the loss of a bony tail and teeth. The center of aerodynamic lift acts at the hand wing anterior to the humeral
axis, causing substantial torsional loading which is effectively resisted by the bone’s large polar moment
of inertia achieved by its expanded, hollow circular cross-sectional shape. (C) The pennate pectoralis is
largely comprised of fast-twitch oxidative fibers that allow for rapid shortening and sustained flight.
Fast-glycolytic fibers are recruited for non-sustainable burst flight activity (From: Biewener 2022).
| Avian personalities -- Personalities are general properties of humans and other animals. Different personality traits are phenotypically correlated, and heritabilities of personality traits have been reported in humans and various animals. In Great Tits, consistent heritable differences have been found in relation to exploration, which is correlated with various other personality traits. van Oers et al. (2004) examined whether or not risk-taking behavior is part of these avian personalities. They found that (1) risk-taking behavior is repeatable and correlated with exploratory behavior in wild-caught hand-reared birds, (2) in a bi-directional selection experiment on ‘fast’ and ‘slow’ early exploratory behavior, bird lines tend to differ in risk-taking behavior, and (3) within-nest variation of risk-taking behavior is smaller than between-nest variation. To show that risk-taking behavior has a genetic component in a natural bird population, van Oers et al. (2004) bred Great Tits in the lab and artificially selected ‘high’ and ‘low’ risk-taking behavior for two generations. They found a realized heritability of 19.3% for risk-taking behavior. With these results, the authors show that risk-taking behavior is linked to exploratory behavior, and provide evidence for the existence of avian personalities. | Risk-taking behavior was also found to be correlated with other aspects of avian personality. Novelty, exploration and risk-taking behaviors seem to be traits of the personality concept, which is in line with the results of other studies on personalities. Risk-taking behavior is known to influence life-history decisions, and evidence is also accumulating that other personality traits affect reproduction, survival and dispersal. In sum, birds have genetically determined personalities that can be observed in a variety of ecological circumstances. |
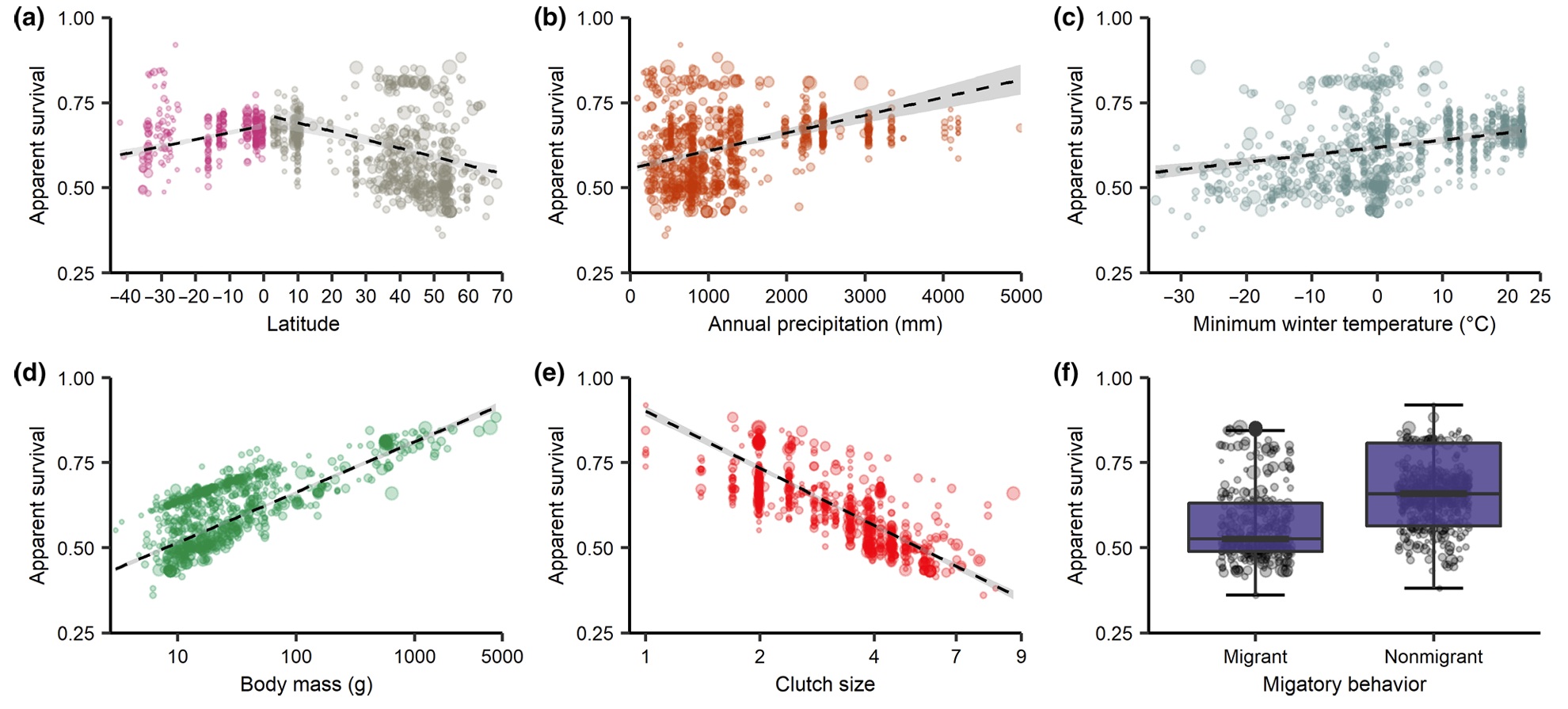
Birds do typically live longer than similar-sized mammals. However, what factors influence how longs birds live given their size, life-history
strategies, and geographical location? A meta-analysis of 949 estimates from 204 studies of avian survival revealed that a latitudinal survival gradient
exists in the northern hemisphere, is less apparent or absent for
southern hemisphere species, and that differences between passerines and non-passerines
largely
drive these trends. Although extrinsic factors related to climate were poor
predictors of apparent survival compared to latitude, the relationship
between apparent
survival and latitude is strongly mediated by intrinsic traits, i.e., large-bodied species and species
with smaller clutch
sizes had the highest apparent survival. Overall, differences
among intrinsic traits and whether species were passerines or non-passerines are more
important than latitude and
its underlying climatic factors in explaining global patterns of apparent avian survival (From: Scholer et al. 2020).
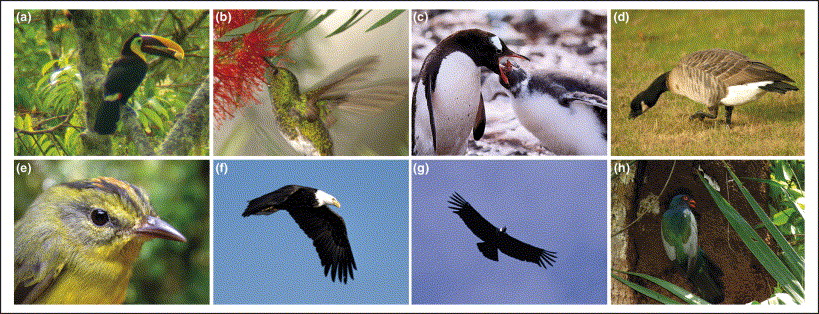
Examples of the eight main types of avian ecosystem service providers. (a) Seed disperser: Black-mandibled Toucan, Ramphastos ambiguus (Las Cruces, Costa Rica). (b) Pollinator: Snowy-bellied Hummingbird, Amazilia edward (Las Cruces, Costa Rica). (c) Nutrient depositor: Gentoo Penguin, Pygoscelis papua (Port Lockroy, Antarctica). (d) Grazer: Cackling Goose, Branta hutchinsii (California, USA). (e) Insectivore: Golden-crowned Warbler, Basileuterus culicivorus (Las Cruces, Costa Rica). (f) Raptor: Bald Eagle, Haliaeetus leucocephalus (Alaska, USA). (g) Scavenger: Andean Condor, Vultur gryphus (Patagonia, Chile). (h) Ecosystem engineer: Slaty-tailed Trogon, Trogon massena (Pipeline Road, Panama). From: Sekercioglu (2006).
The ecological functions of birds (Sekercioglu 2006) -- Birds are mobile links that are crucial for maintaining ecosystem function, memory and resilience. Avian ecological functions encompass all three major linkages: genetic, resource and process. Seed-dispersing frugivores and pollinating nectarivores are genetic linkers that carry genetic material from one plant to another or to habitat that is suitable for regeneration, respectively. Piscivorous birds are resource linkers whose droppings transport aquatic nutrients to terrestrial environments. Grazers, such as geese, and predatory birds, such as insectivores and raptors are trophic process linkers that influence plant, invertebrate and vertebrate prey populations, respectively. Ecosystem engineers, such as woodpeckers are non-trophic process linkers that modify their environment by physically transforming materials from one state to another. Mobile link categories are not mutually exclusive. Birds, particularly colonial species (e.g. social weavers Philetairus socius) and woodpeckers, can modify their environment substantially by constructing nests, which are often used by a variety of other species. Thus, many bird species are both trophic and physical process linkers. Piscivorous bird colonies can carry out all of these linkages as these birds can consume fish, deposit nutrients, engineer ecosystems via burrow construction and even disperse seeds that are adhered to their feet.
Birds also benefit humans by providing important ecosystem services such as: provisioning services via game meat for food, down for garments and guano for fertilizer; regulating services by scavenging carcasses and waste, by controlling populations of invertebrate and vertebrate pests, by pollinating and dispersing the seeds of plants; cultural services, as exemplified by the prominent roles of birds in art and religion and by the billions of dollars spent on birdwatching; and supporting services by cycling nutrients and by contributing to soil formation (Sekercioglu 2006).
Literature Cited
Abourachid et al. 2023. An upright life, the postural stability of birds: a tensegrity system. Journal of the Royal Society Interface 20: 20230433.
Alonso, P. D., A. C. Milner, R. A. Ketcham, M. J. Cookson & T. B. Rowe. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430: 666 - 669.
Austad, S. N. 1997. Birds as models of aging in biomedical research. ILAR Journal 38.
Benton, M. J. 2014. How birds became birds. Science 345: 508-509.
Bokma, F. 2002. A statistical test of unbiased evolution of body size in birds. Evolution 56: 2499-2504.
Biewener, A. A. 2022. Biomechanics of avian flight. Current Biology 32: R1110-R1114.
Bokma, F. 2004. Why most birds are small - a macro-ecological approach to the evolution of avian body size. Ph.D. dissertation, University of Oulu, Oulu, Finlan
Bormashenko, E., Y. Bormashenko, T. Stein, and G. Whyman. 2007. Why do pigeon feathers repel water? Hydrophobicity of pennae, Cassie-Baxter wetting hypothesis and Cassie-Wenzel capillary-induced wetting transition. Journal of Colloid and Interface Science 311: 212-216.
Brusatte, S. L. 2025. The lost long tail of early bird evolution. Nature 638: 323-324.
Callaway, E. 2014. Rival species recast significance of 'first bird.' Nature 516: 18-19.
Carril et al. 2024. Evolution of avian foot morphology through anatomical network analysis. Nature Communications 15: 9888.
Carney, R. M., J. Vinther, M. D. Shawkey, L. D'Alba, and J. Ackermann. 2012. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nature Communications 3:637.
Choiniere, J. N., X. Xu, J. M. Clark, C. A. Forster, Y. Guo, and F. Han. 2010. A basal Alvarezsauroid theropod from the Early Late Jurassic of Xinjiang, China. Science 327: 571-574.
Clark, J. and K. Middleton. 2006.Bird evolution. Current Biology 16: R350-R354.
Claramunt, S., and J. Cracraft. 2015. A new time tree reveals Earth history's imprint on the evolution of modern birds. Science Advances 11: e1501005.
Codd, J. R., D. F. Boggs, S. F. Perry, and D. R. Carrier. 2005. Activity of three muscles associated with the uncinate processes of the giant Canada Goose Branta canadensis maximus. Journal of Experimental Biology 208: 849 -857.
Codd, J. R., P. L. Manning, M. A. Norell, and S. F. Perry. 2008. Avian-like breathing mechanics in maniraptoran dinosaurs. Proceedings of the Royal Society B 275: 157-161.
Cuthill, I.C., W. L. Allen, K. Arbuckle, B. Caspers, G. Chaplin, M. E. Hauber, G. E. Hill, N. G. Jablonski, C. D. Jiggins, A. Kelber, and J. Mappes. 2017. The biology of color. Science 357: 0221.
Davis, P. G. 1998. The bioerosion of bird bones. Int. J. Osteoarcheology 7:388-401.
Deeming, D. C. 2023. Allometry of the pectoral flight muscles in birds: Flight style is related to variability in the mass of the supracoracoideus muscle. Journal of Zoology 319: 264–273.
Degernes, L. A. and A. Feduccia. 2001. Tenectomy of the supracoracoideus muscle to deflight Pigeons (Columba livia) and Cockatiels (Nymphicus hollandicus). Journal of Avian Medicine and Surgery 15: 10–16.
Dove, C. J., A. M. Rijke, X. Wang, and L. S. Andrews. 2007. Infrared analysis of contour feathers: the conservation of body heat radiation in birds. Journal of Thermal Biology 32: 42-46.
Fajardo, R. J., E. Hernandez, and P. M. O’Connor. 2007. Postcranial skeletal pneumaticity: a case study in the use of quantitative microCT to assess vertebral structure in birds. Journal of Anatomy 211: 138-147.
Feduccia, A. 2005. Mesozoic aviary takes form. Proceedings of the National Academy of Science USA 102: 18998-19002.
Feduccia, A. and J. Nowicki. 2002. The hand of birds revealed by early ostrich embryos. Naturwissenschaften 89: 391–393.
Fields et al. 2018. Early Evolution of Modern Birds Structured by Global Forest Collapse at the End-Cretaceous Mass Extinction. Current Biology 28: 1825-1831.
Gatesy, S. M. and K. P. Dial. 1996. From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution 50: 2037-2048.
Geist, N. R. and A. Feduccia. 2000. Gravity-defying behaviors: identifying models for protoaves. American Zoologist 40: 664-675.
Galis, F., M. Kundrát, and B. Sinervo. 2002. An old controversy solved: bird embryos have five fingers. Trends in Ecology and Evolution 18:7-9.
Glen, C. L. and M. B. Bennett. 2007. Foraging modes of Mesozoic birds and non-avian theropods. Current Biology 17: R911-R912.
Guglielmo1, C. G., T. Piersma, and T. D. Williams. 2001. A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. Journal of Experimental Biology 204: 2683-2690.
Heilmann, G. 1926. The origin of birds. Witherby, London.
Huxley, T. H. 1868. On the animals which are most nearly intermediate between the birds and reptiles. Ann. Mag. Nat. Hist. 2:66–75.
James, F. C., and J. A. Pourtless IV. 2009. Cladistics and the origin of birds: a review and two new analyses. Ornithological Monographs No. 66.
Ji, Q., M. A. Norell, K.-Q. Gao, S.-A. Ji, and D. Ren. 2001. The distribution of integumentary structures in a feathered dinosaur. Nature 410: 1084-1088.
Kovacs, C. E. and R. A. Meyers. 2000. Anatomy and histochemistry of flight muscles in a wing-propelled diving bird, the Atlantic Puffin, Fratercula arctica. Journal of Morphology 244: 109-125.
Krishnan, A. 2023. Biomechanics illuminates form–function relationships in bird bills. Journal of Experimental Biology 226 (Suppl_1): jeb245171.
Larsson, H.C.E. and G.P. Wagner. 2002. Pentadactyl ground state of the avian wing. J. Exp. Zool. (Mol. Dev. Evol.) 294: 146–151.
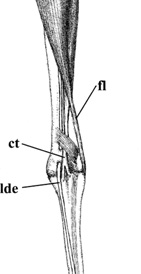
Linn, K. A., A. S. Templer, J. R. Paul-Murphy, R. T. O'Brien, B. K. Hartup, & J. A. Langenberg. 2003. Ultrasonographic imaging of the Sandhill Crane (Grus canadensis) intertarsal joint. Journal of Zoo and Wildlife Medicine 34: 144-152.
Louchart, A., and L. Viriot. 2011. From snout to beak: the loss of teeth in birds. Trends in Ecology and Evolution 26: 663-673.
Lowi-Merri et al. 2021. The relationship between sternum variation and mode of locomotion in birds. BMC Biology 19: article number 165.
Maderspacher, F. 2022. Flightless birds. Current Biology 32: R1042–R1172.
Makovicky, P. J., and L. E. Zanno. 2011. Theropod diversity and the refinement of avian characteristics. In: Living dinosaurs: the evolutionary history of modern birds (G. Dyke and G. Kaiser, eds.), pp. 9-29. John Wiley and Sons, New York, NY.
Marshall, M. 2026. How did birds evolve? The answer is wilder than anyone thought. Nature, https://www.nature.com/articles/d41586-026-00076-z.
Marek, R. D. 2023. A surrogate forelimb: Evolution, function and development of the avian cervical spine. Journal of Morphology 284: e21638.
Meyers, R. A. 2019. Comparative anatomy of the postural mechanisms of the forelimbs of birds and mammals. Journal of Ornithology 160: 869–882.
O'Connor, J. 2025. Archaeopterx 35: R643-R644.
O'Connor, J. 2022. Enantiornithes. Current Biology 32: pR1166-R1172.
O'Connor et al. 2025. Chicago Archaeopteryx informs on the early evolution of the avian bauplan. Nature 641: 1201–1207.
O'Connor et al. 2026. Avian features of Archaeopteryx feeding apparatus reflect elevated demands of flight. The Innovation 7: 2101086.
Organ, C. L., A. M. Shedlock, A. Meade, M. Pagel, and S. V. Edwards. 2007. Origin of avian genome size and structure in non-avian dinosaurs. Nature 446: 180-184.
Ostrom, J. H. 1976. Archaeopteryx and the origin of birds. Biological Journal of the Linnean Society 8:91–182.
Padian, K. 1996. Early bird in slow motion. Nature 382:400-401.
Prum, R. O. 1999. Development and evolutionary origin of feathers: Journal of Experimental Zoology 285: 291–306.
Prum, R. O., S. Andersson, and R.H. Torres. 2003. Coherent scattering of ultraviolet light by avian feather barbs. Auk 120:163-170.
Prum, R. O., J. S. Berv, A. Dornburg, D. J. Field, J. P. Townsend, E. M. Lemmon, and A. R. Lemmon. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526: 569-573.
Rezende et al. 2020. Shrinking dinosaurs and the evolution of endothermy in birds. Science Advances 6 : eaaw4486.
Scholer et al. 2020. A meta-analysis of global avian survival across species and latitude. Ecology Letters 23: 1537-1549.
Schweitzer, M. H., Z. Suo, R. Avci, J. M. Asara, M. A. Allen, F. T. Arce, and J. R. Horner. 2007. Analyses of Soft Tissue from Tyrannosaurus rex Suggest the Presence of Protein. Science 316: 277-280.
Seebacher, F. 2003. Dinosaur body temperatures: the occurrence of endothermy and ectothermy. Paleobiology 29: 105-122.
Sekercioglu, D. H. 2006. Increasing awareness of avian ecological function. Trends in Ecology and Evolution 21:464-471.
Shin et al. 2024. Fast ground-to-air transition with avian-inspired multifunctional legs. Nature 636: 86–91.
Stoessel, A., and M. S. Fischer. 2012. Comparative intralimb coordination in avian bipedal locomotion. Journal of Experimental Biology 215: 4055-4069.
Sullivan, T. N., B. Wang, H. D. Espinosa, and M. A. Meyers. 2017. Extreme lightweight structures: avian feathers and bones. Materials Today 20: 377-391.
Terrill, R. S., and A. J. Shultz. 2023. Feather function and the evolution of birds. Biological Reviews 98: 540-566.
van den Hout, P. J., T. Piersma, A. Dekinga, S. K. Lubbe, and G. H. Visser. 2006. Ruddy Turnstones Arenaria interpres rapidly build pectoral muscle after raptor scares. Journal of Avian Biology 37: 425-430.
van Oers, K., P.J. Drent, P. De Goede and A.J. van Noordwijk. 2004. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proceedings of the Royal Society B 271: 65-73.
Wagner, G. P. and J.A. Gauthier. 1999. A solution to the problem of the homology of the digits in the avian hand. Proc. Natl Acad. Sci. USA 96: 5111–5116.
Xu, X., Z. Zhou, R. Dudley, S. Mackem, C. M. Chuong, G. M. Erickson, and D. J. Varricchio. 2014. An integrative approach to understanding bird origins. Science 346: 1253293.
Xu, X., Z. Zhou, and X. Wang. 2000. The smallest known non-avian theropod dinosaur. Nature 408: 705 - 708.
Yu, M., Z. Yue, P. Wu, D.-Y. Wu, J.-A. Mayer, M. Medina, R.B. Widelitz, T.-X. Jiang, and C.-M. Chuong. 2004. The developmental biology of feather follicles. Int. J. Dev. Biol. 48: 181-191.
Zeffer et al. 2003. Functional correlation between habitat use and leg morphology in birds (Aves). Biological Journal of the Linnean Society 79: 461–484.
Zhang, F., S. L. Kearns, P. J. Orr, M. J. Benton, Z. Zhou, D. Johnson, X. Xu, and X. Wang. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463: 1075-1078.
Zhang, F., Z. Zhou, X. Xu, X. Wang, and C. Sullivan. 2008. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455: 1105-1108.
Zhou, Z. 2004. The origin and early evolution of birds: discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften 91: 455-471.
Zimmer, K. 1935. Beitrage zur mechanik der atmung bei den v ögeln in stand und flug. Aufgrund anatomischer-physiologisher und experimenteller Studien. Zoologica 88:1 -69.